Minimally invasive right hepatectomy for living liver donation: a systematic review of the literature
Introduction
Liver transplantation (LT) represents the mainstay treatment for patients with the end-stage liver disease (1). Nevertheless, the long-lasting imbalance between graft availability and the increasing number of patients waiting for a LT requires the development of new strategies aimed at increasing the donor pool (2). Living donor liver transplantation (LDLT) has emerged as one of the possible solutions for this problem (3). The first reported LDLT series were adult-to-child transplants based on the use of a left lateral liver graft (4,5). Rapidly, adult-to-adult LDLT was also introduced in the clinical practice, with the first series reported in Hong Kong (6). However, performing a right hepatectomy (RH) in a healthy individual should represent a challenge, with non-neglectable reported percentages of donor post-operative discomfort, morbidity, and even mortality (7).
A way for reducing all of these shortcomings has been connected with the introduction of mini-invasive surgery. After the first case of laparoscopic cholecystectomy reported in 1987 (8), mini-invasive approach rapidly became a reality also in the setting of liver surgery. The first laparoscopic anatomic hepatectomies were reported in 1996 (9,10). After the first pioneering laparoscopic major hepatectomies (11), several series reported structured case-series with results even favoring laparoscopy respect to open approaches (12-15). Growing evidence has been reported that mini-invasive liver surgery (MILS) is a feasible approach for a great number of liver diseases, as clearly reported in the Consensus Conferences of Louisville 2008 (16), Morioka 2014 (17) and Southampton 2017 (18). For example, laparoscopy is considered today as the approach of choice for performing a left lateral sectionectomy. However, although great benefits should be surely taken into account in using MILS for liver surgery, its use in the setting of living donation still raises several concerns about donor safety and graft integrity (19). These doubts are even increased in the specific setting of adult-to-adult right lobe donation (20).
A systematic review of the literature has been done specifically investigating the results of the laparoscopic right lobe donation, mainly looking at the different surgical methodologies adopted and the donor complication rates.
Methods
Search strategy
A systematic search was done concerning relevant studies focused on the use of MILS in the setting of living donor-related RH. The search strategy was done following the PRISMA guidelines, as well as PRISMA for abstracts (21). A search of the electronic databases MEDLINE-PubMed was conducted using the following research terms: (laparoscopy[th] OR laparoscopic[th] OR minimally invasive[th] OR hybrid[th] OR hand-assisted[th]) AND (hepatectomy[th] OR liver resection[th] OR hepatic resection[th]) AND (living donor[th] OR living donation[th] OR liver donor[th]).
Studies published before January 1, 2018, were taken into consideration.
Screening process
The present qualitative systematic review included a priori search criteria of journal articles among adult (age ≥18 years) human patients. Studies were limited to the English language.
All the studies in which a RH for living donation performed with any kind of mini-invasive approach (i.e., pure laparoscopic, hybrid, hand-assisted, laparoscopic-assisted, robotic) were selected. Exclusion criteria were: (I) studies reporting donor RH with open technique; (II) studies focused on laparoscopic RH not performed for living donation; (III) papers lacking sufficient statistical details; (IV) review articles; (V) nonclinical studies; (VI) expert opinions or commentaries; (VII) letters to editor; and (VIII) conference summaries. Case reports and case series were considered for the analysis, due to the scarcity of reported cases in the literature.
Study selection
Two reviewers (FG and QL) independently screened the identified studies and their extracted data. In case of disagreement, the paper was discussed by all the authors.
Quality assessment
Selected studies were reviewed based on the representativeness of the study population, comparability of cohorts, adequate assessment of outcomes, sufficient length of follow-up, adequacy of follow-up, and source of study funding. The quality of the papers was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS): studies with scores >6 were defined as high-quality studies (22).
Donor characteristics were collected in Table 1. The following features were reported: first author’s name, year of publication, reference, number of reported donors, type of incision for graft extraction, kind of surgical approach, age, gender, body mass index (BMI), operation time, blood loss during operation in mL, estimated future liver remnant (FLR) expressed in percentage, graft weight in grams, graft-to-recipient weight ratio (GRWR), the incidence of complications, the number of complications stratified according to the Clavien-Dindo classification, and the hospital length of stay in days.
Table 1
| Author [year] (Ref) | N | Incision | Approach | Age (years) | Gender (M/F) | BMI | Operation time (min) | Blood loss (mL) | Estim FLR (%) | Graft weight (g) | GRWR | Complication, n [%] | CD | LOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eguchi [2018] ( |
43 | Minilalap | Hybrid | 44 [24–64] | 9/34 | NA | 410 [314–581] | 600 [130-1,800] | NA | NA | NA | 5 [12] | NA | 14 [8–30] |
| Han [2017] ( |
1 | Pfannestiel | Pure | 19 | 0/1 | NA | 410 | NA | 608 | 0.81 | None | – | NA | |
| Suh [2018] ( |
45 | Pfannestiel | Pure | 33 | 26/19 | 24 | 331 | 436 | 34 | 714 | 1.30 | 4 [9] | I=1/II=2/III=1 | 8 |
| Kitajima [2017] ( |
41 | Minilap | Hybrid | 52 [20–67] | 15/26 | 22 [17–29] | 431 [310–651] | 201 [10–1,559] | NA | 668 [460–1,100] | NA | 9 [22] | I=5/II=4 | 12 [8–27] |
| Hong [2017] ( |
1 | Pfannestiel | Pure | 50 | 1/0 | 26 | 443 | – | NA | 1146 | 1.88 | None | – | 8 |
| Li [2017] ( |
1 | Pfannestiel | Pure | 40 | 1/0 | 480 | – | NA | 615 | 0.85 | None | – | 7 | |
| Takahara [2017] ( |
25 | Minilap | Assisted | 36±10 | 5/20 | 22±2 | 380±45 | 268±194 | NA | 651 | NA | 4 [16] | I=1/II=1/III=2 | 9±2 |
| 5 | Pfannestiel | Pure | 40±9 | 2/3 | 22±2 | 480±54 | 91±69 | NA | 669 | NA | 1 [20] | III=1 | 9±2 | |
| Kim [2017] ( |
3 | Pfannestiel | Pure | 23 | 0/1 | 17 | 502 | 250 | NA | 580 | 0.93 | None | – | 7 |
| Pfannestiel | Pure | 21 | 0/1 | 22 | 427 | 200 | NA | 540 | 0.78 | None | – | 8 | ||
| Pfannestiel | Pure | 33 | 0/1 | 18 | 447 | 270 | NA | 550 | 0.89 | None | – | 7 | ||
| Hong [2017] ( |
9 | Pfannestiel | Pure | 33±11 | 5/4 | 24±1 | 251±50 | 420±183 | NA | NA | NA | NA | NA | 8±1 |
| Shen [2016] ( |
28 | Minilap | Hybrid | 40±11 [22–63] | 15/13 | 23±2 [17–25] | 386±50 [300–508] | 384±180 [200–1,000] | NA | 634±124 [409–869] | 1.00±0.12 [0.80–1.23] | 5 [18] | I=2/II=2/III=1 | 7±3 [6–14] |
| Chen [2016] ( |
13 | Pfannestiel | Robotic | NA | 4/9 | 22 [17–27] | 596 [353–753] | 169 [50–500] | 37 [31–44] | 618 [350–820] | 1.25 [0.89-1.96] | 1 [8] | III=1 | 7 [6-8] |
| Rotellar [2017] ( |
5 | Pfannestiel | Pure | 29 | 1/0 | 26 [21–28] | 480 | <100 | 38 | 1046 | 1.69 | 1 [20] | I=1 | 4 |
| Pfannestiel | Pure | 27 | 0/1 | 450 | <200 | 41 | 791 | 0.96 | NA | NA | 4 | |||
| Pfannestiel | Pure | 48 | 1/0 | 480 | <100 | 36 | 973 | 1.24 | 1 [20] | I=1 | 5 | |||
| Pfannestiel | Pure | 21 | 1/0 | 420 | <100 | 32 | 1,433 | 1.50 | NA | NA | 5 | |||
| Pfannestiel | Pure | 51 | 1/0 | 476 | <100 | 41 | 775 | 1.58 | NA | NA | 3 | |||
| Suh [2016] ( |
2 | Pfannestiel | Pure | 57 | 0/1 | NA | 408 | NA | 40 | 601 | 0.80 | None | – | 9 |
| Pfannestiel | Pure | 40 | 0/1 | 409 | 39 | 669 | 1.10 | None | – | 8 | ||||
| Li [2016] ( |
1 | Pfannestiel | Pure | 47 | 1/0 | 24 | 540 | 350 | 36 | 823 | 1.42 | None | – | 8 |
| Chen [2016] ( |
1 | Pfannestiel | Pure | 19 | 1/0 | NA | 415 | 150 | 36 | 940 | 1.11 | 1 [100] | I=1 | 6 |
| Brustia [2015] ( |
2 | Pfannestiel | Pure | 50±1 | 0/2 | 21±5 | 480 | 125±106 | NA | 590±127 | NA | None | – | 8 |
| Soyama [2015] ( |
25 | Minilap | Hybrid | 41 [21–65] | 12/13 | 22 [17–29] | 411 [324–581] | 600 [130–1900] | NA | NA | NA | 2 [8] | I=1/III=1 | NA |
| Makki [2014] ( |
26 | Minilap | Assisted | 28±9 | 13/13 | 24±4 | 703±124 | 337±89 | NA | 756±88 | NA | 4 [16] | I=3/III=1 | NA |
| Choi [2014] ( |
2 | Pfannestiel | Pure | 25±7 [14–44] | 1/24 | 21±4 [17–29] | 678±110 | 1,000±283 | 35±5 [27–43] | 617±113 [430–845] | NA | 35* | I=32/II=1/III=2 | 12 [10–14] |
| 9 | Minilap | Hybrid | 484±104 | 308±133 | 9 [8-18] | |||||||||
| 14 | Transverse incision | Assisted | 334±60 | 266±127 | 9 [8–24] | |||||||||
| Zhang [2014] ( |
25 | Minilap | Hybrid | 32±9 [22–57] | 13/12 | 24±3 | 386±47 | 378±113 | NA | 630±129 | 0.99±0.20 | 4 [16] | I=3/II=1 | 7±1 |
| Rotellar [2013] ( |
1 | Pfannestiel | Pure | 29 | 1/0 | NA | 480 | <100 | 39 | 1,046 | 1.67 | None | – | 4 |
| Ha [2013] ( |
20 | Minilap | Assisted | 25±6 | 11/9 | 23±4 | 336±94 | 290±67 | NA | 725±136 | NA | 1 [5] | I=1 | 11±3 |
| Soubrane [2013] ( |
1 | Pfannestiel | Pure | 50 | 0/1 | NA | 480 | 100 | 44 | 620 | 0.91 | None | – | 7 |
| Nagai [2012] ( |
28 | Minilap | Hybrid | 34±10 | 15/13 | 24±3 | 371±52 | 212±114 | 36±12 | 915±361 | NA | 7 [20] | II=4/III=3 | 6±1 |
| Choi [2012] ( |
20 | Minilap | Assisted** | 32±10 | 23/17 | 23±3 | 279±72 | 450±316 | NA | NA | NA | 6 [30] | I=1/III=5 | 12±5 |
| 40 | Minilap | Assisted | 30±10 | 12/8 | 24±3 | 384±42 | 870±653 | 6 [15] | I=4/III=2 | 12±3 | ||||
| Giulianotti [2012] ( |
1 | Minilap | Robotic | 53 | 1/0 | NA | 480 | 350 | NA | 1008 | 1.02 | None | – | 5 |
| Thenappan [2011] ( |
15 | Minilap | Assisted | 34±9 | 7/8 | NA | 320±73 | 1033±1096 | NA | NA | NA | 9 [60] | II=2/III=7 | 6±2 |
| Baker [2009] ( |
33 | Minilap | Assisted | 37±10 | 15/18 | 26±4 | 265±48 | 417±217 | NA | 900±215 | 1.17±0.31 | 7 [21] | I=5/II=2 | 5±1 |
| Suh [2009] ( |
9 | Pfannestiel | Pure | NA | 0/1 | 17 | 765 | NA | NA | NA | 0.95 | 1 [11] | I=4/II=1/III=1 | 10 |
| Pfannestiel | Pure | 0/1 | 19 | 898 | 1.05 | 1 [11] | 14 | |||||||
| Minilap | Assisted | 0/1 | 19 | 575 | 0.91 | 1 [11] | 9 | |||||||
| Minilap | Assisted | 0/1 | 27 | 505 | 1.25 | 1 [11] | 12 | |||||||
| Minilap | Assisted | 0/1 | 29 | 460 | 0.94 | None | 9 | |||||||
| Minilap | Assisted | 0/1 | 22 | 310 | 1.25 | 1 [11] | 9 | |||||||
| Minilap | Assisted | 0/1 | 18 | 545 | 0.74 | None | 8 | |||||||
| Minilap | Assisted | 0/1 | 19 | 495 | 0.82 | 1 [11] | 17 | |||||||
| Minilap | Assisted | 0/1 | 24 | 535 | 0.84 | None | 8 | |||||||
| Suh [2008] ( |
2 | Pfannestiel | Pure | 25 | 0/1 | NA | 765 | NA | NA | 560 | 0.95 | None | – | 10 |
| Pfannestiel | Pure | 24 | 0/1 | 898 | 550 | 1.09 | None | 14 | ||||||
| Koffron [2006] ( |
1 | Minilap | Assisted | 32 | 0/1 | NA | 235 | 150 | NA | 825 | NA | None | – | 7 |
| Kurosaki [2006] ( |
3 | Minilap | Assisted | 39±12 | 2/1 | 23±3 | 363±3 | 302±91 | NA | 420±2.9 | 0.72±0.24 | 1 [33.3] | I=1 | 11±3 |
*Total number of complications (more than one complication for each case); **, single-port. Ref, reference; N, number; M, male; F, female; BMI, body mass index; FLR, future liver remnant; GRWR, graft-to-recipient weight ratio; CD, Clavien-Dindo; LOS, length of stay; NA, not available.
Recipient characteristics were collected in Table 2. The following data were collected: first author’s name, reference, year of publication, number of reported recipients, gender, age, underlying liver disease, BMI, the model for end-stage liver disease (MELD) score, complications, and hospital length of stay.
Table 2
| Author | Year | N | Gender (M/F) | Age | Underlying liver disease | BMI | MELD | Complications | LOS |
|---|---|---|---|---|---|---|---|---|---|
| Eguchi ( |
2017 | 43 | NA | NA | NA | NA | NA | NA | NA |
| Han ( |
2016 | 1 | 1/0 | 47 | HBV | NA | NA | NA | NA |
| Suh ( |
2017 | 45 | 30/15 | 53 | HBV 33, HCV 4, alcohol 2, others 6, HCC 32 | 24 | 11 | Intra-abdominal bleeding 3, intra-abdominal fluid collection 4, wound problem 2, hepatic artery problem 1, portal vein or hepatic vein problem 4, biliary problem 1, cardiac problem 1, pulmonary problem 2, gastrointestinal problem 1 | 21 |
| Kitajima ( |
2017 | 76 | 40/36 | 57 [23–69] | NA | 23 [15–32] | 16 [6–40] | Arterial complications 1, portal venous thrombosis 2, biliary leak 5; biliary stricture 5 | NA |
| Hong ( |
2017 | 1 | NA | NA | NA | NA | NA | NA | NA |
| Li ( |
2017 | 1 | 1/0 | 36 | HBV-HCC | NA | 11 | NA | NA |
| Takahara ( |
2017 | 40 | 22/18 | 51±2 | Cholestatic disease 8, HCC 24, vascular disease 1, neoplastic disease 2, acute liver failure 5 | NA | 18±10 | Reoperation 9, hepatic artery thrombus 1, portal vein thrombus/stenosis 2, out flow block 3, biliary complications 4, renal dysfunction 6, rejection 7; mortality (1 month) 2, mortality (3 months) 5 | NA |
| 14 | 9/5 | 52±3 | Cholestatic disease 2, HCC 10, neoplastic disease 1, others 1 | NA | 15±5 | Reoperation 3, hepatic artery thrombus 1, biliary complications 2, renal dysfunction 4, rejection 2; mortality (1 month) 2, mortality (3 months) 2 | NA | ||
| Kim ( |
2017 | 1 | 1/0 | 20 | Wilson | 20 | 8 | NA | 16 |
| 1 | 1/0 | 48 | Alcohol | 25 | 18 | NA | 18 | ||
| 1 | 0/1 | 54 | HBV | 22 | 21 | NA | 24 | ||
| Hong ( |
2017 | 9 | NA | NA | NA | NA | NA | NA | NA |
| Shen ( |
2016 | 28 | NA | NA | NA | NA | NA | NA | NA |
| Chen ( |
2016 | 13 | NA | NA | NA | NA | NA | Artery thrombosis 1, biliary complication 1 | NA |
| Rotellar ( |
2017 | 5 | 4/1 | 67 [44–69] | Primary biliary cholangitis 1, alcohol 1, HCC 3 | NA | 10 [9–16] | Biliary leak 1, biliary stenosis 2, arterial stenosis 1 | NA |
| Suh ( |
2016 | 2 | 1/0 | 62 | HBV and HCC | 24 | 11 | None | 12 |
| 1/0 | 42 | HBV and HCC | 21 | 12 | None | 12 | |||
| Li ( |
2016 | 1 | 0/1 | NA | Sclerosing cholangitis and HCC | NA | 8 | None | NA |
| Chen ( |
2016 | 1 | 1/0 | NA | HBV and HCC | NA | 10 | Pneumonia | NA |
| Brustia ( |
2015 | 2 | NA | NA | NA | NA | NA | NA | NA |
| Soyama ( |
2015 | 25 | NA | NA | NA | 25 [20–36] | 15 [7–40] | NA | NA |
| Makki ( |
2014 | 26 | NA | NA | HCV 10, HBV 5, HCC 3, Alcohol 3, others 8 | NA | 19±7 | Biliary leak 1, biliary stricture 1 | NA |
| Choi ( |
2014 | 2 | NA | NA | NA | NA | NA | NA | NA |
| 9 | NA | NA | NA | NA | NA | NA | NA | ||
| 14 | NA | NA | NA | NA | NA | NA | NA | ||
| Zhang ( |
2013 | 25 | 20/5 | 43±8 | HBV and HCC 21, fulminant hepatitis 3, Budd-Chiari syndrome 1 | NA | 13±6 | Biliary stricture 1, hepatic artery thrombosis 1, intra-abdominal bleeding 1, intra-abdominal abscesses 1, pulmonary infection 1 | NA |
| Rotellar ( |
2013 | 1 | 1/0 | 69 | Cryptogenetic cirrhosis with HCC | NA | 15 | Pseudomonas aeruginosa pneumonia | NA |
| Ha ( |
2013 | 20 | NA | NA | NA | NA | NA | NA | NA |
| Soubrane ( |
2013 | 1 | 0/1 | 47 | Primary biliary cholangitis | NA | 22 | None | 15 |
| Nagai ( |
2012 | 28 | 11/17 | NA | HCV 11, alcoholic cirrhosis 2, autoimmune hepatitis 2, primary biliary cholangitis 3, primary sclerosing cholangitis 5, other 5 | NA | 11±6 | Biliary stricture/leak 4, Hepatic artery thrombosis/stricture 2, Hepatic vein stricture 2, Intra-abdominal abscess 2 | 14±8 |
| Choi ( |
2012 | 40 | NA | NA | NA | NA | NA | NA | NA |
| 20 | NA | NA | NA | NA | NA | NA | NA | ||
| Giulianotti ( |
2011 | 1 | 1/0 | 61 | HCV | NA | NA | None | 8 |
| Thenappan ( |
2011 | 15 | NA | NA | NA | NA | NA | NA | NA |
| Baker ( |
2009 | 33 | 24/9 | 52±14 | HCV 3, HCC 6, cholangiocarcinoma 3 | 26±6 | 12±4 | NA | NA |
| Suh ( |
2009 | 9 | 1/0 | 51 | HBV and HCC | NA | 15 | Stroke (IIIA), biliary stricture (IIIA) | 32 |
| 0/1 | 62 | HBV and HCC | NA | 10 | Bile leak (II), biliary stricture (IIIA) | 39 | |||
| 1/0 | 62 | HBV and HCC | NA | 12 | Biliary stricture (IIIA) | 17 | |||
| 0/1 | 46 | Budd-Chiari syndrome and HCC | NA | 10 | Biliary stricture (IIIA) | 24 | |||
| 1/0 | 58 | HBV and HCC | NA | 15 | None | 17 | |||
| 0/1 | 14 | Biliary atresia | NA | 21 | Seizure (I), ascending cholangitis (II) | 26 | |||
| 1/0 | 59 | HBV | NA | 16 | Bile stricture (IIIA) | 21 | |||
| 0/1 | 55 | HBV and HCC | NA | 9 | Tremor (I), bile leak (IIIA), HA stenosis (II), PV thrombosis (IIIB) | 61 | |||
| 0/1 | 51 | HBV | NA | 31 | None | 26 | |||
| Suh ( |
2008 | 2 | 1/0 | NA | HCC | NA | NA | None | NA |
| 0/1 | NA | NA | NA | NA | None | NA | |||
| Koffron ( |
2006 | 1 | 1/0 | NA | Progressive sclerosing cholangitis | NA | NA | None | NA |
| Kurosaki ( |
2005 | 3 | 1/2 | 49±14 | Viral liver cirrhosis and HCC 3 | NA | 24±8 | NA | NA |
N, number; M, male; F, female; BMI, body mass index; MELD, model for end-stage liver disease; LOS, length of stay; NA, not available; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Statistical analysis
Continuous variables were reported as mean ± standard error or median and ranges. Dichotomous variables were reported as number and percentages. A univariate logistic regression analysis was performed investigating the risk of post-operative donor complication. OR and 95% confidence intervals (95% CI) were reported. OR was considered statistically significant when the P was <0.05. OR and 95% CI >1 revealed a higher risk of postoperative complication, whereas a result <1 had the opposite meaning. SPSS statistical package version 23.0 (SPSS Inc., Chicago, IL, USA) was used.
Results
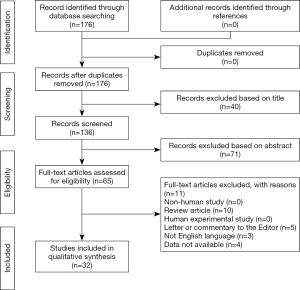
The selection process of the articles is explained in Figure 1.
As for the selection process according to the PRISMA guidelines, the various examined databases provided a total of 176 articles to screen. After carefully checking for the references of these articles, no papers more were identified reaching the characteristics established for the present study. Consequently, 176 articles were initially screened. After reading the title and the abstract, 111 articles were removed. Of the remaining 65 papers, 33 were not considered eligible after full-text evaluation. Eventually, 32 articles were identified, with a total of 501 investigated cases (Tables 1,2).
As for the quality of the reported studies, all the examined articles were only case reports or case series. Consequently, a NOS value was impossible to be correctly established, thus underlying the poor overall quality of the studies focused on this topic.
Only 8 (25.0%) articles coming from Western centers were reported, with only 59/501 (11.8%) cases performed.
As for the type of MILS, pure laparoscopic RH and robotic hepatectomy were done in 84 (16.8%) and 14 (2.8%) donors, respectively. Hybrid or assisted procedures were done in 199 (39.7%) and 204 (40.7%) cases, respectively. The type of incision done for extracting the graft was a mini-laparotomy in 381 (76.0%) cases, a transverse incision in 14 (2.8%) subjects, and Pfannenstiel incision in 106 (21.2%) donors, respectively. A total of 285 (56.9%) donors were females. In the 464 cases in which the postoperative course was exhaustively described for each patient, a total of 85 (18.3%) subjects experiencing at least one complication were reported. Twenty-six (5.6%) individuals had a grade III according to the Clavien-Dindo classification: no cases of organ dysfunction or death were experienced (Table 1).
After stratification of the entire population according to the type of laparoscopic approach adopted (pure-robotic vs. hybrid-assisted), it was interesting to observe that hybrid-assisted cases presented an increased risk of experiencing any complication after RH, with an OR of 2.53 (95% CI: 1.22–5.24; P value=0.01).
Recipient-related characteristics were less extensively reported (Table 2): only 17 articles reported post-operative recipient course, with a total of 113/291 (38.8%) cases reporting any complication.
Discussion
MILS for living donation has been introduced in the clinical practice with the intent to reduce the impact on donor’s life. In fact, laparoscopy aims to reach several positive aspects, like (I) minimizing tissue trauma, (II) reducing postoperative pain, (III) decreasing morbidity and mortality rates, (IV) obtaining better cosmetic results, (V) consenting a faster return to work and normal physical activities.
Some evidence exists about the benefit of performing a laparoscopic left lateral sectionectomy for adult-to-pediatric transplantation (55,56).
However, no clear evidence exists on the benefits of performing such a procedure in the setting of adult-to-adult RH donation.
A recent meta-analysis comparing laparoscopy-assisted vs. open right lobe donation reported that the first approach was connected with a reduced intraoperative blood loss (weighted mean difference =−58 mL, 95% CI: −94–−21; P value=0.002). However, although the complication rate was inferior in both hybrid left and right lobe procedures respect to the open procedures (relative risk =0.70; 95% CI: 0.51–0.94; P value=0.02), in the subgroup analysis, comprehending only RHs no differences were observed (relative risk =0.95; 95% CI: 0.63–1.43; P value=0.80). Similar negative results were also found just investigating Clavien-Dindo grades ≥3 (57).
In the present analysis, no comparison has been made between open and laparoscopic procedures. On the opposite, the systematic collection of all the worldwide reported laparoscopic RH consented to perform an analysis aimed at comparing “pure laparoscopic” approaches (namely, robotic- or pure laparoscopy) with hybrid ones (namely hand-assisted or laparoscopic-assisted procedures). As for the overall incidence of intraoperative complications, a significant benefit in favor of pure approach was reported, with a 2.5-fold increased risk of any complication after hybrid approach. Such a result is significant, mainly because the previous meta-analysis investigating hybrid vs. open approach showed a slight reduction of the risk in case of the laparoscopic procedure. Indeed, we can postulate that a sort of gradient exists concerning improved safety for the donor, passing from open approach to hybrid to pure laparoscopic procedure.
Some doubts should be reported regarding the here observed results. Hybrid approach presents, in fact, some apparent benefits: for example, manual hand manipulation in the abdominal cavity gives to the surgeon tactile feedback of the liver. Moreover, the possibility to rapidly extract the graft is connected with a reduced warm ischemia time. We can only suppose that pure laparoscopy gives better results because it is typically approached in centers with very high laparoscopic expertise. As a consequence, the reduced number of complications is not directly connected with a real superiority of the procedure, but to the fact that centers at the beginning of their learning curve for major hepatectomy start their programs using hybrid approaches. Thus, we can postulate that a progressive reduction of the complications will be reported in the next future also in case of assisted procedures.
Of interest, no severe donor morbidity or mortality was reported in the reported series: in other terms, zero on 501 cases of donor death were reported respect to the 23/1,153 (0.2%) cases previously published in the open series (7).
Unfortunately, it was impossible to analyze if the different laparoscopic surgical approaches in the donor were also connected with different outcomes in the recipient: in fact, the vast majority of the series selected in the present study did not report detailed information on postoperative complications in the recipients. Consequently, it was not possible to see in detail if pure laparoscopy was connected with the more complex management of the dissection area.
Another critical shortcoming of the study was the tiny number of studies comparing the different laparoscopic techniques and the fact that possible heterogeneities should exist among donors treated with different approaches.
Conclusions
Laparoscopic RH for living donation is a safe procedure. After 501 reported procedures, no deaths have been described. Pure laparoscopic approaches look to consent a lower risk of donor complication respect to hybrid ones. More studies comparing the different laparoscopic approaches with the open procedure are required.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037ls.2018.04.09). QL serves as an unpaid editorial board member of Laparoscopic Surgery from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Starzl TE. The long reach f liver transplantation. Nat Med 2012;18:1489-92. [Crossref] [PubMed]
- Ghinolfi D, Lai Q, Pezzati D, et al. Use of elderly donors in liver transplantation: A paired-match analysis at a single center. Ann Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Lieber SR, Schiano TD, Rhodes R. Should living donor liver transplantation be an option when deceased donation is not? J Hepatol 2018;68:1076-82. [Crossref] [PubMed]
- Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet 1989;2:497. [Crossref] [PubMed]
- Strong RW, Lynch SV, Ong TH, et al. Successful liver transplantation from a living donor to her son. N Engl J Med 1990;322:1505-7. [Crossref] [PubMed]
- Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997;226:261-9. [Crossref] [PubMed]
- Cheah YL, Simpson MA, Pomposelli JJ, et al. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl 2013;19:499-506. [Crossref] [PubMed]
- Cuschieri A, Dubois F, Mouiel J, et al. The European experience with laparoscopic cholecystectomy. Am J Surg 1991;161:385-7. [Crossref] [PubMed]
- Azagra JS, Goergen M, Gilbart E, et al. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc 1996;10:758-61. [Crossref] [PubMed]
- Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery 1996;120:468-75. [Crossref] [PubMed]
- Hüscher CG, Lirici MM, Chiodini S, et al. Current position of advanced laparoscopic surgery of the liver. J R Coll Surg Edinb 1997;42:219-25. [PubMed]
- Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62. [Crossref] [PubMed]
- Descottes B, Lachachi F, Sodji M, et al. Early experience with laparoscopic approach for solid liver tumors: initial 16 cases. Ann Surg 2000;232:641-5. [Crossref] [PubMed]
- Shimada M, Hashizume M, Maehara S, et al. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc 2001;15:541-4. [Crossref] [PubMed]
- Gigot JF, Glineur D, Santiago Azagra J, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg 2002;236:90-7. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The International Position on Laparoscopic Liver Surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second International Consensus Conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Cauchy F, Schwarz L, Scatton O, et al. Laparoscopic liver resection for living donation: where do we stand? WJG 2014;20:15590-8. [Crossref] [PubMed]
- Kim KH, Yu YD, Jung DH, et al. Laparoscopic living donor hepatectomy. Korean J Hepatobiliary Pancreat Surg 2012;16:47-54. [Crossref] [PubMed]
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: Reporting systematic reviews in journal and conference abstracts. PLoS Med 2013;10:e1001419 [Crossref] [PubMed]
- Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:iii-x, 1-173. [Crossref] [PubMed]
- Eguchi S, Soyama A, Hara T, et al. Standardized hybrid living donor hemihepatectomy in adult-to-adult living donor liver transplantation. Liver Transpl 2018;24:363-8. [Crossref] [PubMed]
- Han YS, Ha H, Kwon HJ, et al. Pure laparoscopic donor right hepatectomy in a living donor with type 3a biliary variation: A case report. Medicine (Baltimore) 2017;96:e8076 [Crossref] [PubMed]
- Suh KS, Hong SK, Lee KW, et al. Pure laparoscopic living donor hepatectomy: Focus on 55 donors undergoing right hepatectomy. Am J Transplant 2018;18:434-43. [Crossref] [PubMed]
- Kitajima T, Kaido T, Iida T, et al. Short-term outcomes of laparoscopy-assisted hybrid living donor hepatectomy: a comparison with the conventional open procedure. Surg Endosc 2017;31:5101-10. [Crossref] [PubMed]
- Hong SK, Suh KS, Kim HS, et al. Pure 3D laparoscopic living donor right hemihepatectomy in a donor with separate right posterior and right anterior hepatic ducts and portal veins. Surg Endosc 2017;31:4834-35. [Crossref] [PubMed]
- Li J, Huang J, Wu H, et al. Laparoscopic living donor right hemihepatectomy with venous outflow reconstruction using cadaveric common iliac artery allograft: Case report and literature review. Medicine (Baltimore) 2017;96:e6167 [Crossref] [PubMed]
- Takahara T, Wakabayashi G, Nitta H, et al. The First Comparative Study of the Perioperative Outcomes Between Pure Laparoscopic Donor Hepatectomy and Laparoscopy-Assisted Donor Hepatectomy in a Single Institution. Transplantation 2017;101:1628-36. [Crossref] [PubMed]
- Kim KH, Kang SH, Jung DH, et al. Initial Outcomes of Pure Laparoscopic Living Donor Right Hepatectomy in an Experienced Adult Living Donor Liver Transplant Center. Transplantation 2017;101:1106-10. [Crossref] [PubMed]
- Hong SK, Lee KW, Kim HS, et al. Optimal bile duct division using real-time indocyanine green near-infrared fluorescence cholangiography during laparoscopic donor hepatectomy. Liver Transpl 2017;23:847-52. [Crossref] [PubMed]
- Shen S, Zhang W, Jiang L, et al. Comparison of Upper Midline Incision With and Without Laparoscopic Assistance for Living-Donor Right Hepatectomy. Transplant Proc 2016;48:2726-31. [Crossref] [PubMed]
- Chen PD, Wu CY, Hu RH, et al. Robotic liver donor right hepatectomy: A pure, minimally invasive approach. Liver Transpl 2016;22:1509-18. [Crossref] [PubMed]
- Rotellar F, Pardo F, Benito A, et al. Totally Laparoscopic Right Hepatectomy for Living Donor Liver Transplantation: Analysis of a Preliminary Experience on 5 Consecutive Cases. Transplantation 2017;101:548-54. [Crossref] [PubMed]
- Suh KS, Hong SK, Yi NJ, et al. Pure 3-dimensional laparoscopic extended right hepatectomy in a living donor. Liver Transpl 2016;22:1431-6. [Crossref] [PubMed]
- Li H, Wei Y, Li B, et al. The First Case of Total Laparoscopic Living Donor Right Hemihepatectomy in Mainland China and Literature Review. Surg Laparosc Endosc Percutan Tech. 2016;26:172-5. [Crossref] [PubMed]
- Chen KH, Huang CC, Siow TF, et al. Totally laparoscopic living donor right hepatectomy in a donor with trifurcation of bile duct. Asian J Surg 2016;39:51-5. [Crossref] [PubMed]
- Brustia R, Komatsu S, Goumard C, et al. From the left to the right: 13-year experience in laparoscopic living donor liver transplantation. Updates Surg 2015;67:193-200. [Crossref] [PubMed]
- Soyama A, Takatsuki M, Hidaka M, et al. Hybrid procedure in living donor liver transplantation. Transplant Proc 2015;47:679-82. [Crossref] [PubMed]
- Makki K, Chorasiya VK, Sood G, et al. Laparoscopy-assisted hepatectomy versus conventional (open) hepatectomy for living donors: when you know better, you do better. Liver Transpl 2014;20:1229-36. [Crossref] [PubMed]
- Choi Y, Yi NJ, Lee KW, et al. Section 17. Laparoscopic and minimal incisional donor hepatectomy. Transplantation 2014;97:S69-75. [Crossref] [PubMed]
- Zhang X, Yang J, Yan L, et al. Comparison of laparoscopy-assisted and open donor right hepatectomy: a prospective case-matched study from china. J Gastrointest Surg 2014;18:744-50. [Crossref] [PubMed]
- Rotellar F, Pardo F, Benito A, et al. Totally laparoscopic right-lobe hepatectomy for adult living donor liver transplantation: useful strategies to enhance safety. Am J Transplant 2013;13:3269-73. [Crossref] [PubMed]
- Ha TY, Hwang S, Ahn CS, et al. Role of hand-assisted laparoscopic surgery in living-donor right liver harvest. Transplant Proc 2013;45:2997-9. [Crossref] [PubMed]
- Soubrane O, Perdigao Cotta F, Scatton O. Pure laparoscopic right hepatectomy in a living donor. Am J Transplant 2013;13:2467-71. [Crossref] [PubMed]
- Nagai S, Brown L, Yoshida A, et al. Mini-incision right hepatic lobectomy with or without laparoscopic assistance for living donor hepatectomy. Liver Transpl 2012;18:1188-97. [Crossref] [PubMed]
- Choi HJ, You YK, Na GH, et al. Single-port laparoscopy-assisted donor right hepatectomy in living donor liver transplantation: sensible approach or unnecessary hindrance? Transplant Proc 2012;44:347-52. [Crossref] [PubMed]
- Giulianotti PC, Tzvetanov I, Jeon H, et al. Robot-assisted right lobe donor hepatectomy. Transpl Int 2012;25:e5-9. [Crossref] [PubMed]
- Thenappan A, Jha RC, Fishbein T, et al. Liver allograft outcomes after laparoscopic-assisted and minimal access live donor hepatectomy for transplantation. Am J Surg 2011;201:450-5. [Crossref] [PubMed]
- Baker TB, Jay CL, Ladner DP, et al. Laparoscopy-assisted and open living donor right hepatectomy: a comparative study of outcomes. Surgery 2009;146:817-23. [Crossref] [PubMed]
- Suh KS, Yi NJ, Kim T, et al. Laparoscopy-assisted donor right hepatectomy using a hand port system preserving the middle hepatic vein branches. World J Surg 2009;33:526-33. [Crossref] [PubMed]
- Suh KS, Yi NJ, Kim J, et al. Laparoscopic hepatectomy for a modified right graft in adult-to-adult living donor liver transplantation. Transplant Proc 2008;40:3529-31. [Crossref] [PubMed]
- Koffron AJ, Kung R, Baker T, et al. Laparoscopic-assisted right lobe donor hepatectomy. Am J Transplant 2006;6:2522-5. [Crossref] [PubMed]
- Kurosaki I, Yamamoto S, Kitami C, et al. Video-assisted living donor hemihepatectomy through a 12-cm incision for adult-to-adult liver transplantation. Surgery 2006;139:695-703. [Crossref] [PubMed]
- Almodhaiberi H, Kim SH, Kim KH. Totally laparoscopic living donor left hepatectomy for liver transplantation in a child. Surg Endosc 2018;32:513. [Crossref] [PubMed]
- Park JI, Kim KH, Lee SG. Laparoscopic living donor hepatectomy: a review of current status. J Hepatobiliary Pancreat Sci 2015;22:779-88. [Crossref] [PubMed]
- Zhang B, Pan Y, Chen K, et al. Laparoscopy-Assisted versus Open Hepatectomy for Live Liver Donor: Systematic Review and Meta-Analysis. Can J Gastroenterol Hepatol 2017;2017:2956749
Cite this article as: Giovanardi F, Lai Q, Melandro F, Larghi Laureiro Z, Di Tomaso A, Curci FP, Hassan R, Rossi M, Mennini G. Minimally invasive right hepatectomy for living liver donation: a systematic review of the literature. Laparosc Surg 2018;2:17.