
A multi-institutional analysis of minimally invasive liver resections
Introduction
Hepatobiliary surgeons continue to struggle over the optimal role for minimally invasive liver surgery in the management of hepatic tumors (1-3). The laparoscopic approach has slowly replaced a segment of open liver resection (OLR). Despite great enthusiasm, some centers have less than a 10% adoption rate while others report an aggressive 80% incidence of minimally invasive liver resections (MILR). This rate of laparoscopic adoption has varied significantly across the international spectrum as well, varying from 43% in select groups within the European Union to less than 20% in the Americas (4). Since the earliest reports of MILR in the early 1990s, multiple authors have advocated numerous approaches, techniques and surgical devices (5-7). Subsequently, there have been three significant international consensus meetings held to evaluate, define, and recommend standardize guidelines for approaches in MILR (1-3).
As the minimally invasive approach gains greater adoption and acceptance, techniques and outcomes must be critically analyzed through multi-institutional clinical trials and large propensity case series comparing open, laparoscopic and robotic approaches to liver resections (8-10). Initial reports, series and clinical trials have confirmed innumerable valuable improvements to patient outcomes while maintaining the oncologic integrity of the operation (1-3). The most recent innovation in liver surgery has been the introduction of robotic liver surgery, where there is a paucity of published literature (11-13). The objective of this study was to evaluate the operative experience of three senior high volume hepatobiliary surgeons over a 16-year study period. The primary endpoint of the study was major complications, operating time, blood loss, and 90-day mortality.
Methods
A retrospective chart review from three high volume surgeons of all consecutive liver resections was performed. Each institution maintained a prospective database of MILR from 2000 to 2016. The study received approval from the Institutional Board Review Committee of Tulane University (No. 351684-OTH) with a waiver of written informed consent. MILR was defined as laparoscopic and robotic-assisted resections. OLR were recorded. Exclusion criteria were patients less than 18 years of age. Patient demographics including race, age, gender, tumor characteristics were recorded. Intra-operative characteristics including surgeon, operative time, and estimated blood loss (EBL) were also obtained. Major liver resections were identified as right or left lobectomies or tri-segmentectomies. The primary endpoint of the study was to evaluate 90-day patient mortality. Other patient outcomes measured included hospital length of stay (HLOS) and patient re-admission. Major complications (bile leak, takeback operation, post-op bleeding, post-operative infection, wound dehiscence, respiratory difficulty, myocardial infarction, and renal dysfunction) were also measured and compared between the two groups.
Data from all three institutions were pooled for analysis. Univariate analysis for statistical significance was performed using Student’s t test for continuous variables and Fisher’s exact test for categorical variables. Kaplan-Meier survival curves were generated to compare 90-day patient mortality between MILR and OLR patients. A binary logistic regression was used to evaluate several variables (type of surgical procedure, major liver resection, patient age, gender, malignancy, EBL) on patient mortality. Data were analyzed using GraphPad software (version 5, La Jolla, CA) for univariate analysis and SPSS IBM software for multivariate analysis (version 24, Armonk, NY).
Results
Study population
A cohort of 1,323 patients were included with 746 OLR (56.4%) and 577 MILR [530 laparoscopic, 40.1% and 47 robotic liver resections (RLRs), 3.6%]. The number of MILRs increased significantly during the study period (0.5%, year 2000, vs. 40.5%, year 2016, P<0.001) as shown in Figure 1.
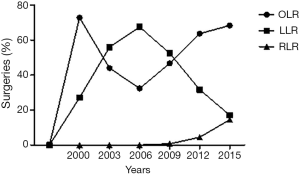
Surgical procedures
Data was obtained from three different hepatobiliary surgeons with a significant amount of MILR had significantly decreased EBL (634.2±33.4 vs. 275.9±18.4 mL, P<0.0001). The average case length for MILR liver resections was less compared to OLR (258.3±2.9 vs. 288.3±4.2 min, P<0.001). These results are shown in Table 1.
Table 1
| Demographics | Total (n=1,323) | OLR (n=746) | MILR (n=577) | P value |
|---|---|---|---|---|
| Age, mean (SEM), years | 56.8 (0.4) | 58.2 (0.5) | 54.9 (0.6) | <0.0001 |
| Male gender, n (%) | 604 (45.7) | 375 (50.3) | 229 (39.7) | 0.0002 |
| Race, n (%) | ||||
| Caucasian | 955 (72.2) | 537 (72.0) | 418 (72.4) | 0.90 |
| African American | 240 (18.1) | 106 (14.2) | 134 (23.2) | <0.0001 |
| Asian | 71 (5.4) | 59 (7.9) | 12 (2.1) | <0.0001 |
| Other races | 57 (4.3) | 44 (5.9) | 13 (2.3) | 0.0010 |
| Operative details | ||||
| Oncologic resection, n (%) | 932 (70.4) | 644 (86.3) | 288 (49.9) | <0.0001 |
| Major resection, n (%) | 526 (39.8) | 354 (47.5) | 172 (29.8) | <0.0001 |
| EBL, mean (SEM), mL | 477.1 (21.0) | 634.2 (33.4) | 275.9 (12.1) | <0.0001 |
| Operation time, mean (SEM), min | 276.9 (3.1) | 288.3 (4.2) | 258.3 (2.9) | <0.0001 |
SEM, standard error of mean; EBL, estimated blood loss.
Patient outcomes
The average hospital length of stay was higher in the OLR group (8.7±0.3 vs. 4.2±0.2 days, P<0.001). The incidence of post-op re-admissions was also significantly higher in the OLR group compared to MILR (10.2% vs. 4.0%, P<0.0001).
Complications
The overall incidence of post-operative complications was significantly higher in the OLR cohort (35.5% vs. 16.1%, P<0.0001). Post-operative infections were higher in the OLR group (8.0% vs. 1.4%, P<0.0001). No difference in bile leak (P=0.42) or take back to the operating room (P=0.29) was found between the techniques. The incidence of the 5-year disease recurrence was found to be significantly higher in the open group (42.5% vs. 27.9%, P<0.001). Results are presented in Table 2.
Table 2
| Outcome | Total (n=1,323) | OLR (n=746) | MILR (n=577) | P value |
|---|---|---|---|---|
| Hospital LOS, mean (SEM), days | 6.9 (0.2) | 8.7 (0.3) | 4.2 (0.2) | <0.0001 |
| Readmission, n (%) | 99 (7.5) | 76 (10.2) | 23 (4.0) | <0.0001 |
| Take back surgery, n (%) | 15 (1.1) | 11 (1.5) | 4 (0.7) | 0.20 |
| Bile leak, n (%) | 41 (3.1) | 26 (3.5) | 15 (2.6) | 0.42 |
| Post-operative infection, n (%) | 68 (5.1) | 60 (8.0) | 8 (1.4) | <0.0001 |
| Major post-operative complication, n (%) | 358 (27.1) | 265 (35.5) | 93 (16.1) | <0.0001 |
| Post-operative bleed, n (%) | 33 (2.5) | 24 (3.2) | 9 (1.6) | 0.07 |
| 90-day mortality, n (%) | 24 (1.8) | 18 (2.4) | 6 (1.0) | 0.09 |
SEM, standard error of mean; LOS, length of stay.
Patient survival
Overall patient survival for the study cohort was 75.6% with an average follow-up length of 1.7±0.1 years. When comparing 90-day patient mortality between the groups, there was no significant difference between the OLR and MILR (2.4% vs. 1.0%, P=0.09). Kaplan-Meier 90-day survival curve was generated for OLR and MILR and is shown in Figure 2. Log rank test did not show a significant difference in 90-day mortality between the groups [hazard ratio (HR) 0.55; 95% CI, 0.2–1.6, P=0.54].

Multivariate analysis for mortality
MILR as a risk factor for 90-day death was analyzed using a binary logistic regression to control for possible cofounders (Table 3). MILR was not found to increase the risk of 90-day mortality [odds ratio (OR) 0.6; 95% CI, 0.2–1.6, P=0.3].
Table 3
| Independent variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| MILR | 0.6 | 0.2–1.6 | 0.300 |
| Age | 1.000 | 1.000–1.048 | 0.384 |
| EBL | 1.000 | 1.000–1.001 | 0.247 |
| Male gender | 1.3 | 0.6–3.1 | 0.500 |
| Oncologic resection | 1.8 | 0.5–6.7 | 0.400 |
| Major resection | 0.9 | 0.4–2.2 | 0.900 |
MILR, minimally invasive liver resection; EBL, estimated blood loss.
RLR
A sub-group analysis was performed on RLRs, which represented 3.6% (n=47/1,323) of total resections performed. The first RLR for this data set was performed in 2009 with increasing numbers observed 2014–2016 (23.4%, 34.0%, and 25.5%, respectively). Univariate analysis of RLRs compared to the large pool of laparoscopic liver resections (LLRs) did not find any significant differences in patient outcomes or complications compared to LLRs (Table 4). Interestingly, RLR was performed for a higher percentage of oncologic resections compared to LLR (80.9% vs. 47.2%, P<0.0001). Kaplan-Meier survival curve for RLR vs. LLR is shown in Figure 2B (HR 0.23; 95% CI, 0.04–1.26, P=0.10).
Table 4
| Variables | Robotic (n=47) | Laparoscopic (n=530) | P value |
|---|---|---|---|
| Age, mean (SEM), years | 59.6 (1.8) | 54.6 (0.6) | 0.02 |
| Male gender, n (%) | 23 (48.9) | 209 (39.4) | 0.22 |
| Caucasian, n (%) | 33 (70.2) | 392 (74.0) | 0.61 |
| Cancer resection, n (%) | 38 (80.9) | 250 (47.2) | <0.0001 |
| Hospital LOS, mean (SEM), days | 4.3 (0.5) | 4.2 (0.2) | 0.88 |
| EBL, mean (SEM), mL | 200.6 (31.0) | 281.4 (19.6) | 0.22 |
| Bile leak, n (%) | 0 | 15 (2.8) | 0.62 |
| Post-operative infection, n (%) | 1 (2.1) | 7 (1.3) | 0.50 |
| Major post-operative complication, n (%) | 7 (14.9) | 88 (16.6) | 1.00 |
| Post-operative bleed, n (%) | 2 (4.3) | 8 (1.5) | 0.19 |
| Overall mortality, n (%) | 4 (8.5) | 53 (10.0) | 1.00 |
| 90-day mortality, n (%) | 1 (2.1) | 5 (0.9) | 0.40 |
SEM, standard error of mean; LOS, length of stay; EBL, estimated blood loss.
Discussion
MILR continues to gain wide acceptance amongst hepatobiliary surgeons and patients alike. A decade of studies confirmed the benefits of MILR though the benefits of RLR have not been as clear to date. The current study provides further evidence in the hands of early adopters of MILR for the continued proliferation of laparoscopic and RLR in high volume academic specialty centers. Several robotic series have indicated the benefit of robotic platforms including 3D high definition visualization, stable platforms, fourth arm retraction, arm articulation for suturing and newly deployed monopolar devices as well as inline stapling (14,15). Recent upgrade to the Da Vinci Xi platform (Sunnyvale, CA, USA) allows a new approach to targeting and robot placement to minimize robotic arm interference.
A recent study by the French Hepatectomy Study Group analyzed 44,240 liver resections performed in France between 2007 and 2012 (16). The laparoscopic cohort was comprised of 7,881 patients or 17.8% of cases. In this study, the incidence of LLR increased more than open resection (7.0% vs. 1.3%) but most procedures were relegated to minor resections (61.1% vs. 28.9%; P<0.001). This result is in contrast to 15% of major resections undergoing laparoscopic procedures in only 19.5% of hospitals that performed liver resections. The proportion of cases performed laparoscopically was directly inverse to the annual caseload.
In contrast, a group of European Specialized Centers including Ghent, Oslo, Southampton, and Milan did 43% of cases laparoscopically (4). This observation confirms laparoscopic liver surgery is still in the phase of early and interim phase adoption. Clearly, as is documented in our series from three early adopters, a 40–60% utilization of MILR is feasible, but is most reasonably achievable at high volume specialty centers.
The current robotic platform and surgical devices for the performance of MILR resection have significantly evolved over the last decade. The current literature has been limited to lower volume series but each confirming the safety and efficacy of the operation. Several of these series have identified equivalent patient and disease-free survival when deployed for cancer (17,18). Our analysis of the current series confirms that the robotic platform is now robust enough to approach not only minor but major resections with assurance of safety and oncologic integrity. The proliferation of robotic liver surgery like any other early adoption of MILR should proceed first in high-volume centers with experienced hepatobiliary surgeons.
As previously described by several authors, our study did not identify a higher tumor recurrence or an inferior oncologic operation using the MILR approach. A recent review by the Hong Kong group identified an overall patient survival advantage in a laparoscopic resection cohort compared to the open approach for hepatocellular cancer (5-year survival: 83.7% vs. 52.2%) (19). A meta-analysis by Cheng et al. demonstrated similar 5-year patient and disease free survival for MILR compared to open resection to manage metastatic colon cancer to the liver (20). This study further identified an increased R0 rate in the MILR group (OR =0.43, P=0.03). Araki and colleagues then addressed the use of MILR in difficult to access cases including the right posterosuperior segments (VII/VIII) and the caudate (I) confirming an equivalent low R1 rate for these resections (21).
Our study had several limitations. The first limitation was the retrospective nature of the study design. This may have resulted in introduction of possible patient selection bias. In addition, the results presented are the work of three aggressive early adopters of MILR with significant prior hepatobiliary experience. As observed by the French and the European studies, this fact may limit the widespread applicability of these results to other surgeons, especially to low volume centers with less experienced surgeons or even in large volume centers where senior surgeons may be late adopters. In this situation with senior surgeons, robotics may play a more significant role. As was observed in urologic and pelvic surgery, senior surgeons were more apt to adopt minimally invasive approaches using robotic platforms than pure laparoscopic techniques. Finally, more detailed information on the specific anatomic locations and techniques of laparoscopic liver transection and operative outcomes would provide more useful insight into the particular challenges that could be encountered with different types of liver resections.
In conclusion, this large multi-institutional retrospective study of MILR demonstrates that whether laparoscopic or robotic, significant patient benefits including less blood loss, complications, length of stay, readmissions were observed in both groups. Furthermore, no evidence of increased bile leak, re-operation, 90-day mortality or decreased oncologic integrity were apparent. This study provides a snapshot of three large volume centers of experience with the growth of MILR over the past two decades. Future studies are further needed to help solidify the long-term outcomes of MILR, especially in the era of RLRs.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2018.07.01). JFB serves as an unpaid editorial board member of Laparoscopic Surgery from January 2018 to December 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript in ensuring that questions related to the accuracy or integrity of any part of the manuscript are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study received approval from the Institutional Board Review Committee of Tulane University (No. 351684-OTH) with a waiver of written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buell JF, Cherqui D, Geller DA, et al. World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 2018;268:11-8. [PubMed]
- Berardi G, Van Cleven S, Fretland ÅA, et al. Evolution of Laparoscopic Liver Surgery from Innovation to Implementation to Mastery: Perioperative and Oncologic Outcomes of 2,238 Patients from 4 European Specialized Centers. J Am Coll Surg 2017;225:639-49. [Crossref] [PubMed]
- Reich H, McGlynn F, DeCaprio J, et al. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956-8. [PubMed]
- Katkhouda N, Fabiani P, Benizri E, et al. Laser resection of a liver hydatid cyst under video laparoscopy. Br J Surg 1992;79:560-1. [Crossref] [PubMed]
- Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc 1992;6:99.
- Scuderi V, Barkhatov L, Montalti R, et al. Outcome after laparoscopic and open resections of posterosuperior segments of the liver. Br J Surg 2017;104:751-9. [Crossref] [PubMed]
- Martínez-Cecilia D, Cipriani F, Vishal S, et al. Laparoscopic versus open liver resection for colorectal metastases in elderly and octogenarian patients: a multicenter propensity score based analysis of short- and long-term outcomes. Ann Surg 2017;265:1192-200. [Crossref] [PubMed]
- Yoon YI, Kim KH, Kang SH, et al. Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg 2017;265:856-63. [Crossref] [PubMed]
- Daskalaki D, Gonzalez-Heredia R, Brown M, et al. Financial impact of the robotic approach in liver surgery: a comparative study of clinical outcomes and costs between the robotic and open technique in a single institution. J Laparoendosc Adv Surg Tech A 2017;27:375-82. [Crossref] [PubMed]
- Chen PD, Wu CY, Hu RH, et al. Robotic major hepatectomy: is there a learning curve? Surgery 2017;161:642-9. [Crossref] [PubMed]
- Bagante F, Spolverato G, Strasberg SM, et al. Minimally invasive vs. open hepatectomy: a comparative analysis of the national surgical quality improvement program database. J Gastrointest Surg 2016;20:1608-17. [Crossref] [PubMed]
- Giulianotti PC, Sbrana F, Coratti A, et al. Totally robotic right hepatectomy: surgical technique and outcomes. Arch Surg 2011;146:844-50. [Crossref] [PubMed]
- Nota CL, Rinkes IHB, Molenaar IQ, et al. Robot-assisted laparoscopic liver resection: a systematic review and pooled analysis of minor and major hepatectomies. HPB (Oxford) 2016;18:113-20. [Crossref] [PubMed]
- Farges O, Goutte N, Dokmak S, et al. How surgical technology translates into practice: the model of laparoscopic liver resections performed in France. Ann Surg 2014;260:916-21; discussion 921-2. [Crossref] [PubMed]
- Beard RE, Tsung A. Minimally invasive approaches for surgical management of primary liver cancers. Cancer Control. 2017;24:1073274817729234 [Crossref] [PubMed]
- Kim JK, Park JS, Han DH, et al. Robotic versus laparoscopic left lateral sectionectomy of liver. Surg Endosc 2016;30:4756-64. [Crossref] [PubMed]
- Cheung TT, Dai WC, Tsang SH, et al. Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: a propensity analysis at a single center. Ann Surg 2016;264:612-20. [Crossref] [PubMed]
- Cheng Y, Zhang L, Li H, et al. Laparoscopic versus open liver resection for colorectal liver metastases: a systematic review. J Surg Res 2017;220:234-46. [Crossref] [PubMed]
- Araki K, Fuks D, Nomi T, et al. Feasibility of laparoscopic liver resection for caudate lobe: technical strategy and comparative analysis with anteroinferior and posterosuperior segments. Surg Endosc 2016;30:4300-6. [Crossref] [PubMed]
Cite this article as: Smith A, Konstantinidis IT, Fong Y, Martinie J, Iannitti D, Buell JF. A multi-institutional analysis of minimally invasive liver resections. Laparosc Surg 2018;2:35.


