
Laparoscopic radiofrequency of liver metastasis: is its cost effectiveness a good reason to replace hepatic resection?
Hepatic resection (HR) is considered the gold standard for the treatment of colorectal liver metastasis (CRLM), but only a minority of patients (15–20%) is eligible for it because of general health status or presence of comorbidities (1,2). In addition, patients with small single hepatic metastasis could not be ideal surgical candidates due to their localization (3). In the last decade, the integration of surgical procedures, multi-modal treatments [portal embolization, two-stage hepatectomy, associating liver partitioning and portal vein occlusion for staged hepatectomy (ALPPS)…] and the introduction of new anti-angiogenic agents significantly improved resectability rate up to 16–30% (1,4). In order to provide curative treatment to patients who remain irresectable despite of these techniques, alternative thermal ablation (TA) such as radiofrequency ablation (RFA) and microwave ablation (MWA) are increasingly being used (5). In several retrospective publications, these techniques have been shown to prolong survival rate and to improve quality of life for recurrent metastasis, inoperable lesions after complete or partial response to chemotherapy or in patients with resectable multiple CRLM in addiction to surgical treatment (5,6). However, the choice of ablative therapies instead of HR is still controversial in patients eligible for resection. Despite the large number of patients treated with RFA worldwide, a randomized controlled clinical trial comparing this approach with HR has started but final results have not yet been published (7). Recent meta-analyses confirmed that RFA is associated with increased recurrence rates and lower disease-free and overall survival and should therefore only be used for patients who are not good candidates for surgery (8,9). However, these studies collected articles published from 2004 to 2017, including obsolete ablative technologies and patients were almost exclusively submitted to percutaneous approach. In the last years, technology improvements (MWA) (9) and the use of minimally invasive approaches (laparoscopic TA) (10) showed that the local tumor control after ablative therapies is similar to that obtained after HR especially in patients with single small (<3 cm) CRLM potentially resectable.
What do the authors say?
A recent published paper comparing laparoscopic TA with HR demonstrated a cost benefit of laparoscopic RFA over HR in a group of patients with single small (<3 cm) CRLM (11). This benefit translated into a 35% decrease in overall treatment costs when laparoscopic RFA was used as the first line of surgical treatment in the selected patients. This cost benefit could be assumed to last in the follow-up period because laparoscopic RFA has a low rate of local recurrences (9%) in comparison to other publications (also for their institution), probably because they included only patients with single small lesions (<3 cm) (12). Furthermore, Authors outlined that in this study oncological outcomes (overall survival and disease-free survival) were similar between HR and laparoscopic RFA: the median overall survival for HR was 63 and 51 months for ablation (P=0.64), while disease-free survival was 21 and 14 months (P=0.59), respectively. No different rates of postoperative morbidity were found probably due to the fact that the ablation group had higher comorbidity rates (88%) in comparison to HR group (63.5%; P=0.02). On the basis of these results, the authors conclude that laparoscopic RFA represents a valid opportunity in the treatment of CRLM in selected patients, also guaranteeing a cost-savings treatment. This cost savings could also be increased with a more extensive use of MWA which further reduces the rate of local recurrences (12).
What do they not say?
Economic evaluation of surgical or interventional procedures requires complex methods (13): Takahashi’s publication (11) does not detail how the cost analysis was carried out. In addition to this, the authors did not include the follow-up costs: 40 reoperations were necessary in the HR group (with a rate of 0.63 reoperations for each patients) and only 10 in the ablation groups (with a rate of 0.40). However, only 14% of HR patients underwent a new operation for recurrences while 36% of ablation patients needed redo-treatments. Other studies analyzed overall costs including the follow-up period: one of them indicated that for small (<3 cm) solitary metastases, costs of HR treatments were higher than ablation therapies: the additional costs of HR compared to ablation was about £6,290 (13).
In the Takahashi’s paper (11), the choice of laparoscopic TA, rather than HR, was related to patients’ comorbidities only in 7, in other seven cases it was indicated in order to obtain parenchymal preservation[due to deep position, near to Glissonian pedicles (Figure 1)], and in 11 patients it was a patient’s decision: this last setting represents 44% of all indications, higher than the one due to comorbidities.
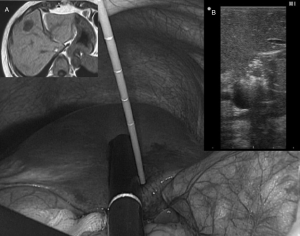
Inclusion criteria in the paper were single lesion with a maximum diameter <3 cm. However, in table 1 the mean tumor size was 1.91±0.09 for HR and 1.81±0.14 for ablation patients. These values suggest that very few patients should have a diameter higher than 25 mm: it would have been very useful to have also the values expressed as median and interquartile range. In fact, it is clear that the smaller the CRLM nodules are, the better the prognosis is. Nonetheless, in our experience in a series of local ablative therapies combined for surgery for multiple CLRM, lesions <20 mm had a local recurrence rates of 11% compared to lesions >20 mm (P=0.009) for which rate is 50% (14). In another study, Authors showed that CRLM bigger than 25 mm at presentation have higher risk of local recurrences after ablative therapy despite preoperative chemotherapy downsizing (15).
In the Takahashi’s publication (11), only RFA technology has been used. Current literature regarding local recurrence rates after MWA versus RFA is still controversial, even if some publications showed some advantages with MWA (3,5,9,12). In our experience, laparoscopic MWA for the treatment of hepatocellular carcinoma obtained lower rates of local tumor progression (8.3%) compared to RFA (21.2%; P=0.034), without any difference in terms of postoperative complications, overall survival and disease-free-survival (16). Similar results have been obtained by Yang et al. (17) for the treatment of CRLM through a laparoscopic access: local tumor progression rates were lower in the MWA group (1.4%) than in the RFA groups (10.2%; P=0.046%). However, these findings did not have a strong impact on overall and disease-free survival rates, similar in both groups.
In the last years, the increasing use of MWA could further improve the local recurrence rate after TA with the hypothetical consequence to reduce the overall costs (reducing the redo-treatments) and to decrease the mortality due to recurrences (3,5,9,12). However, RFA needle’s price is about 900 euros, while MWA’s one is about 1,500 euros. On the other hand, we need to consider that in the HR group Takahashi et al. (11) used high-technology instruments (CUSA, Tissuelink, or Aquamantys) even if 47 out of 65 patients had wedge resection or segmentectomy, that could be performed with a saline-assisted bipolar electrocautery or less expensive devices, even for laparoscopic HR.
What guidelines on RFA for CRLM do not say?
Currently, guidelines for CRLM treatment do not consider TA as an alternative to HR as in the case of hepatocellular carcinoma where EASL Clinical Practice guidelines indicate that: “In patients with very early stage HCC (BCLC-0) RFA in favorable locations can be adopted as first-line therapy even in surgical patients (evidence moderate; recommendation strong)” (18). Guidelines for the use of TA in the treatment of CRLM are summarized in Table 1 (19-25). As you can see, the lack of randomized controlled clinical studies does not allow for a decisive statement in favor of TA, even if the most current guidelines should express themselves decisively in favor of TA associated with HR to allow for parenchymal sparing for patients with multiple/bilobar MRLC. However, there are no precise indications on the ideal size of the lesions suitable for TA treatment (only the recent Chinese guidelines suggest 3 cm as limit for TA) (25).
Table 1
| Guidelines | Year | Comment |
|---|---|---|
| CCO ( |
2013 | TA has been used for unresectable CRLM, sometimes in conjunction with the surgical removal of resectable CRLM, and may have a role in the treatment of other selected patients |
| IKNL ( |
2014 | TA is not an alternative to HR in patients with resectable CLRM, but can be used in selected patients in combination with HR to enable HR. Percutaneous TA can be applied in patients with CRLM who are less suitable for HR by old age, comorbidity, an unfavorable location of the lesion for resection or a history of extensive abdominal surgery |
| KCE ( |
2014 | TA should be considered in addition to HR in patients with CLRM in order to achieve complete response and sufficient residual liver function (strong recommendation) |
| TA is not recommended in patients with unresectable CLRM (strong recommendation) | ||
| SIGN ( |
2016 | Patients with liver and lung metastases should be considered for HR or, in the case of liver disease, |
| Pan-Asian ( |
2018 | In patients with unresectable CRLM only, or oligometastatic disease, TA can be considered. The decision should be taken by a multidisciplinary team based on local experience, tumour characteristics, and patient preference [IV, B] |
| TA can be used in addition to HR with the goal of eradicating all visible metastatic sites [II, B] | ||
| NCCN ( |
2019 | Although HR is the standard approach for the local treatment of resectable CRLM, patients with liver oligometastases can be considered for TA. Evidence on the use of TA as a reasonable treatment option for non-surgical candidates and those with recurrent disease after HR with small CRLM that can be treated with clear margins is growing |
| Chinese ( |
2019 | If CRLM can be removed by R0 resection but the surgery is technically difficult, other means of partial destruction (TA) should be actively used to achieve no evidence of disease. TA can be performed for residual metastatic lesions with a diameter of less than 3 cm |
HR, hepatic resection.
In conclusion, Takahashi’s publication (11) shows that the laparoscopic TA is a viable alternative to HR, with the same oncological results, but with lower costs that do not seem to rise further during the follow-up. However, some biases such as retrospective data collection, heterogeneity of treatments in HR group (laparoscopic or open HR, wedge or major HR) and unclear definition of lesions’ size prevent this excellent publication from having a strong impact on further guidelines. Anyway, this paper agrees with previous studies that reported similar results in terms of local recurrence and survival between RFA and HR (8-10). We are therefore waiting for the final results of a phase III randomized controlled trial (7) still in progress comparing open HR to RFA for isolated CLRM <3 cm in size (laparoscopic approach and MWA are not planned in the study’s design). This trial challenges the ideology that HR is the best treatment of single CLRM, and mirrors the current approach for small hepatocellular carcinoma (18), which have shifted away from HR to TA. However, it will take a while before results will be ready for publication and they may be obsolete in front of technological progresses of laparoscopic HR that can make this approach less invasive, more radical and economically less burdensome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Laparoscopic Surgery. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2019.05.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg 2019;3:50-6. [Crossref] [PubMed]
- Araujo RL, Riechelmann RP, Fong Y. Patient selection for the surgical treatment of resectable colorectal liver metastases. J Surg Oncol 2017;115:213-20. [Crossref] [PubMed]
- Tsitskari M, Filippiadis D, Kostantos C, et al. The role of interventional oncology in the treatment of colorectal cancer liver metastases. Ann Gastroenterol 2019;32:147-55. [PubMed]
- Xu F, Tang B, Jin TQ, et al. Current status of surgical treatment of colorectal liver metastases. World J Clin Cases 2018;6:716-34. [Crossref] [PubMed]
- Schlick CJR, Merkow RP, Bentrem DJ. Nonresectional regional therapies for metastatic colorectal cancer to the liver. J Surg Oncol 2019;119:636-41. [Crossref] [PubMed]
- Sartori S, Tombesi P. Di vece F. Thermal ablation in colorectal liver metastases: lack of evidence or lack of capability to prove the evidence? World J Gastroenterol 2016;22:3511-5. [Crossref] [PubMed]
- Puijk RS, Ruarus AH, Vroomen LGPH, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 2018;18:821. [Crossref] [PubMed]
- van Amerongen MJ, Jenniskens SFM, van den Boezem PB, et al. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases – a meta-analysis. HPB 2017;19:749-56. [Crossref] [PubMed]
- Meijerink MR, Puijk RS, van Tilborg AAJM, et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol 2018;41:1189-204. [Crossref] [PubMed]
- Khajanchee YS, Hammill CW, Cassera MA, et al. Hepatic resection vs minimally invasive radiofrequency ablation for the treatment of colorectal liver metastases. A Markov analysis. Arch Surg 2011;146:1416-23. [Crossref] [PubMed]
- Takahashi H, Akyuz M, Kahramangil B, et al. A comparison of the initial cost associated with resection versus laparoscopic radiofrequency ablation of small solitary colorectal liver metastasis. Surg Laparosc Endosc Percutan Tech 2018;28:371-4. [Crossref] [PubMed]
- Takahashi H, Kahramangil B, Kose E, et al. A comparison of microwave thermosphere versus radiofrequency thermal ablation in the treatment of colorectal liver metastases. HPB 2018;20:1157-62. [Crossref] [PubMed]
- Loveman E, Jones J, Clegg AJ, et al. The clinical effectiveness and cost-effectiveness of ablative therapies in the management of liver metastases: systematic review and economic evaluation. Health Technol Assess 2014;18:vii-viii, 1-283. [Crossref] [PubMed]
- Barabino M, Gatti A, Santambrogio R, et al. Intraoperative local ablative therapies combined with surgery for the treatment of bilobar colorectal liver metastases. Anticancer Res 2017;37:2743-50. [Crossref] [PubMed]
- Benhaim L, El Hajjam M, Malafosse R, et al. Radiofrequency ablation for colorectal cancer liver metastases initially greater than 25 mm but downsized by neo-adjuvant chemotherapy is associated with increased rate of local tumor progression. HPB 2018;20:76-82. [Crossref] [PubMed]
- Santambrogio R, Chiang J, Barabino M, et al. Comparison of laparoscopic microwave to radiofrequency ablation of small hepatocellular carcinoma (<3 cm). Ann Surg Oncol 2017;24:257-63. [Crossref] [PubMed]
- Yang B, Li Y. A comparative study of laparoscopic microwave ablation with laparoscopic radiofrequency ablation for colorectal liver metastasis. JBUON 2017;22:667-72. [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Gallinger S, Biagi JJ, Fletcher GG, et al. Liver resection for colorectal cancer metastases. Curr Oncol 2013;20:e255-65. [Crossref] [PubMed]
- National guideline colon carcinoma. Oncoline. Available online: http://www.oncoline.nl/colorectaalcarcinoom. Assessed 1 August 2014.
- Peeters M, Leroy R, Robays J, et al. Colon Cancer: Diagnosis, Treatment and Follow-Up. Good Clinical Practice (GCP) Brussels: Belgian Health. Care Knowledge Centre (KCE). 2014. KCE Reports 218. D/2014/
10.273/15 . Available online: https://kce.fgov.be/sites/default/files/atoms/files/KCE_218_Colon_cancer.pdf - SIGN 126 • Diagnosis and management of colorectal cancer. A national clinical guideline. December 2011 • Revised August 2016. Available online: https://www.sign.ac.uk/assets/sign126.pdf
- Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO–ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018;29:44-70. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer. Version 1.2019 — March 15, 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- Xu J, Fan J, Cai J, et al. Chinese guidelines for the diagnosis and comprehensive treatment of colorectal liver metastases (version 2018). J Cancer Res Clin Oncol 2019;145:725-36. [Crossref] [PubMed]
Cite this article as: Santambrogio R, Barabino M, Galfrascoli E, Zappa MA. Laparoscopic radiofrequency of liver metastasis: is its cost effectiveness a good reason to replace hepatic resection? Laparosc Surg 2019;3:20.


