Adrenal malignancy: still a contraindication for laparoscopy?
Introduction
Laparoscopic adrenalectomy (LA) has become the gold standard for the resection of benign adrenal masses and has replaced laparotomic open adrenalectomy (OA) as the preferred minimally invasive method for removal of most adrenal tumors (1,2).
However, the role of minimally invasive adrenalectomy remains controversial for the treatment of adrenal malignancies, especially for adrenocortical carcinoma (ACC), a rare but highly aggressive neoplasia (3,4). Some studies, even if including a small series of patients, have reported favorable oncological outcome after LA for ACC (5,6). Other authors, however, have found questionable outcomes of LA in obtaining oncological radicality, that should be achieved in ACC for long-term cure of ACC (4,7). The same controversies exist for the role of LA in adrenal metastases.
This article was aimed to debate the role of minimally invasive adrenalectomy in adrenal malignancies.
The diagnosis of adrenal malignancies: incidentaloma and malignant mass
“Incidentalomas” are defined as asymptomatic adrenal nodules discovered by an imaging study performed for an unrelated reason. They are common, estimated to occur in approximately 3% to 7% of patients older than 60 years old; benign adrenal adenomas are the most common tumor, even if the rate of malignancy is higher in oncologic patients (8-11).
However, the adrenal glands can be affected by malignant or potentially malignant tumors, such as ACC, malignant pheochromocytoma, lymphoma, and metastases (12).
Once radiologically detected, it is important to focus on clinical features associated with adrenal hormonal hypersecretion (hypercortisolism, hyperaldosteronism and pheochromocytoma), and personal and familial history (12).
Further workup is recommended for adrenal masses in patients with known malignancy to differentiate adenomas from metastases (13). On the other side, the management of an incidentally discovered adrenal tumor in patients without a known primary malignancy is controversial (12).
Tumor size has been considered one of the most relevant criteria for malignancy: adrenal masses measuring 1–4 cm in patients without a known history of cancer could be presumed benign (10).
The reported malignancy rate is 6% for lesions with a diameter measuring between 4 and 6 cm and 25% in case of size larger than 6 cm (9,14-16).
Moreover, previous reports revealed that the incidence of malignancies is higher in patients with bilateral adrenal nodules than in those with unilateral nodules (13,17-20).
Generally, malignant adrenal tumors have heterogeneous radiological features with irregular borders. ACCs are often larger (>6 cm) and exhibit rapid dimensions increase and clinical or biochemical features suggesting primary malignancy (e.g., virilization); in contrast, metastatic lesions can present different dimensions and exhibit variable growth patterns (8).
For a more specific radiological description, a 10 Hounsfield unit density threshold has been suggested to diagnose an adenoma at unenhanced computed tomography (CT) (21).
Metastases and ACCs generally enhance rapidly and their washout is prolonged, due to neovascularization of the tumor. Moreover, CT scan provides further critical information about local invasion and distant metastases (16).
Magnetic resonance imaging (MRI) can be particularly useful to differentiate adrenal adenoma from malignant lesions. Adenomas typically present as low signal intensity lesions on both T1- and T2-weighted images, while malignant tumors exhibit hyperintensity in T2-weighted imaging (16,22).
FDG positron emission tomography (PET) might be useful for prediction of malignancy; biopsy should be used only when metastatic disease is presumed in neoplastic patients presenting an enlarging adrenal mass after excluding catecholamine hypersecretion (13).
Minimally invasive adrenalectomy for indeterminate and suspected adrenal lesions
In case of suspected malignancy, the first surgical aim remains the complete removal (16). However, it should be considered that in most cases indeterminate adrenal mass result to be benign at definitive pathology; thus, minimally invasive surgery could be preferable for lesions with atypical imaging features without pathognomonic signs of malignancy. Evident advantages of minimally invasive surgery compared with OA have been highlighted by several reports focusing on immediate perioperative outcomes, postoperative pain and aesthetic results (16,23). The decision to treat these lesions using a minimally invasive approach should take into account the lesion size and features, patient habitus and surgeon experience (16,24).
Management of Adrenal Metastases: indication to operative treatment
Metastatic adrenal involvement should be excluded in oncological patients presenting with an adrenal mass, since they may be found up to 27% of cases in autoptic series (25-27).
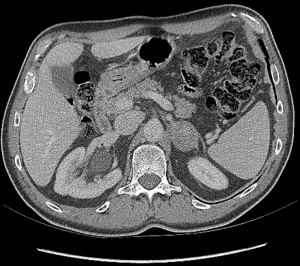
The most frequent primary malignancy metastasizing to adrenals are lung, kidney, breast, gastrointestinal tract carcinomas, and melanoma (28) (Figure 1).
Metastases are defined as “synchronous” if detected at diagnosis of primary tumor or within 6 months, and as “metachronous” if detected after 6 months or more (29).
The presence of synchronous metastases means advanced stage of disease at diagnosis and poor prognosis, requiring additional multiple treatments (30).
Generally, newly diagnosed adrenal lesions in patients with history of malignant tumor should be further investigated by a multidisciplinary team to prevent a decrease of patient life-expectancy (31).
Adrenalectomy for metastatic carcinoma should be performed considering biology of the primary tumor, presence of extra-adrenal disease, patient comorbidity, and efficacy of other therapies (16).
Since the first description in 1982 (32), several studies reported prolonged survival after OA for isolated metastasis, or oligometastatic disease. Current guidelines from the AAES/AACE propose that adrenal metastasectomy is seldom indicated for isolated adrenal metastasis (15,33).
However, the management of adrenal metastasis and primary tumor is still a matter of debate, as the role of the minimally invasive surgical approach (29).
Several studies, even if retrospective, have shown that adrenalectomy can be considered in selected patients with isolated or oligometastatic disease (16,34,35).
Drake et al. (26) have evaluated whether a patient with adrenal metastasis is a good candidate for adrenalectomy. They have elaborated an overview of the largest series from United States and Europe; independently from the surgical approach (even if LA was the most utilized) (33,36-38), they found multiple factors associated with decreased survival after surgery, such as lung primary tumor, disease-free interval <1 year, adrenal metastasis larger than 5 cm, and local retroperitoneal recurrence. Synchronous versus metachronous metastases, extra-adrenal disease, extended adrenalectomy versus isolated adrenalectomy are other predictive factors (26,39).
Notwithstanding all these factors and parameters do not represent absolute exclusion criteria from surgical treatment, nor from a minimally invasive approach (16).
Laparoscopic versus open surgery for adrenal metastases
LA in oncological patients have demonstrated better results in terms of reduced morbidity and shorter length of hospital stay compared with the OA (29).
Recently, laparoscopic indications have been expanded and postoperative complications are indeed the most frequently used marker to define surgery quality. On the other hand, the risk of tumor spillage, port-site metastasis and positive surgical margins with subsequent incomplete resections might negatively impact the oncological outcome in case of adrenalectomy for metastasis; therefore, the role of laparoscopy is controversial in these patients (28,30,34).
Most authors support an open surgical approach for the excision of big lesions; however, the upper threshold proposed for a laparoscopic approach varies among the different studies. It is evident that excision of large lesions can be challenging and at risk for inadequate oncological radicality, but there is no firm evidence that an open approach would gain a better surgical outcome (26,40).
Presently all the data described in literature cannot establish that LA for metastatic disease improves the overall survival of these patients. This is the most significant parameter in terms of oncological outcome and might be evaluated only by prospective randomized trials, that are challenging also from an ethical point of view (26,40-42).
ACC management: the operative treatment
ACC is a rare and aggressive malignant disease, accounting for 0.05–0.2% of all malignancies and less than 5% of all adrenal incidentalomas (38,43).
The 5-year postoperative survival rate ranges from 15% to 60%, and correlates with disease stage at diagnosis (44,45).
Patients affected by ACC can be asymptomatic or can present symptoms of hormone hypersecretion. About 60% of ACC are associated with Cushing’s syndrome; 20–30% with virilization and 10% with feminization. Therefore, a hormonal profile evaluation should be obtained to better characterize the tumor and to provide tumor markers, useful during postoperative follow-up (43,46-48).
Most ACCs are sporadic but may be also associated with numerous hereditary syndromes (Beckwith-Wideman syndrome, multiple endocrine neoplasia type 1, Li-Fraumeni, congenital adrenal hyperplasia, familial adenomatous polyposis, and Lynch syndrome) (38).
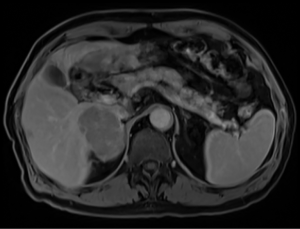
Furthermore, a correct imaging evaluation is essential. CT and MRI are currently the gold standard in the assessment of size, resectability and relationship to other structures (Figure 2). Then a multidisciplinary discussion should be a priority before any treatment if ACC has been suspected (43).
The tumor, lymph node, and metastasis (TNM) classification proposed by the International Union Against Cancer (UICC) and the American Joint Commission on Cancer (AJCC) has been the most widely used classification (49). However, the TNM staging for ACC remains controversial in terms of true prognostic value. The European Network for the Study of Adrenal Tumors (ENSAT) classification can better differentiate from a prognostic point of view stages II and III and for these reasons it has become widely adopted (50). The Weiss score utilizes tumor pathology to assess prognosis (45,46,51,52).
In this context, the ENSAT and the European Society of Endocrine Surgeons (ESES) in a collaborative scientific group, published in 2017 some recommendations and suggestions with the aim to provide standards for the perioperative surgical care of patients with ACC (53) (Table 1).
Table 1
| Multiple hormonal, steroid precursor or sex hormone hypersecretion |
| Radiological signs of malignancy and/or a diameter greater than 6 cm |
| Evidence of local invasion, suspected metastatic lymph nodes, distant metastasis and/or high 18FDG-PET uptake |
| No preoperative biopsy of suspected ACC if surgical radical excision is feasible |
18FDG-PET, fluorine-18 fluorodeoxyglucose positron emission tomography; ACC, adrenocortical carcinoma.
Technical aspects of ACC surgery
Complete resection
The main prognostic factor for ACC is the stage at diagnosis and the completeness of surgical resection. A radical excision can be achieved in early stages of disease (ENSAT stage I–III); complete surgical excision achieves a 5-year survival from 32% to 58%; when incomplete excision occurs, the median survival is generally <1 year. Unfortunately, even after an apparent complete resection, local or distant recurrence may occur in 80% of patients (38,47,54).
Tumor handling
ACC tends also to invade through the tumor capsule; thus, lack of surgical experience may cause tumor rupture and incomplete resection during the handling regardless of the approach. Moreover, higher rates of radical and complete tumor resection and lower rates of positive margins have been reported for ACC initially treated in referral centers; the overall and disease-specific survival is significantly lower when surgery is performed in less experienced centers (46,55-60).
Resection of adjacent organs/vascular structures
Direct invasion into adjacent organs is often reported in ACC, requiring extensive surgery with en bloc resection of involved organs (kidney, liver, spleen, pancreas, stomach, and colon). However, there is no evidence that systematic excision of the adrenal tumor en bloc with kidney could achieve better results when a direct invasion of the renal parenchyma is not established pre or intra-operatively. Sometimes intracaval ACC extension or tumor thrombus are present at diagnosis; however, this situation is not an absolute contraindication to surgery since some satisfactory results may be achieved with the complete removal of the thrombus (47,54).
Lymph node dissection
ACC often spreads via lymphatic drainage, however the role of a regional nodal dissection in ACC, if any, is still unclear (46).
Thus, reported rates of lymph node removal in studies are low (17–30%); standardization of regional lymphadenectomy has been proposed to include first-order drainage stations such as renal hilum, celiac, and paraortic and paracaval lymph nodes above the renal pedicle and ipsilateral to the adrenal gland. A study from the German ACC Registry reporting a series of 283 patients with completely resected ACC showed a significantly reduced risk for tumor recurrence and disease-related death following systematical lymphadenectomy (46,61).
However, the role of lymph node dissection in ACC has not been demonstrated; in most cases lymph nodes dissection is performed based on the intraoperative findings rather than routinely (16,53).
Minimally invasive versus open surgery for ACC
The issue of local recurrence has become the more relevant debate on the surgical approach concerning ACCs, and the first matter of discordance between authors in favor or against minimally invasive surgical approaches. An overview of these studies (6,57,62-71) is provided in Table 2.
Table 2
| Author, year | Study design | Country | Study period | Number of patients | Surgical approach (OA/LA), n [%] | Conversion rate (%) |
|---|---|---|---|---|---|---|
| Brix |
Retrospective case control | Germany | 1996–2009 | 152 | 177 [77]/35 [23] | 34 |
| Cooper |
Retrospective case control | USA | 1993–2012 | 302 | 256 [85]/46 [15] | Not available |
| Donatini |
Retrospective case control | France | 1985–2011 | 34 | 21 [61]/13 [39] | 0 |
| Fossa |
Retrospective case control | Norway | 1998–2011 | 32 | 15 [47]/17 [53] | 11 |
| Gonzalez |
Retrospective case control | USA | 1991–2004 | 139 | 133 [95]/6 [5] | 16 |
| Leboulleux |
Retrospective case control | France | 2003–2009 | 64 | 58 [90]/6 [10] | Not available |
| Lodin |
Retrospective case control | Italy | 1997–2005 | 12 | 7 [58]/5 [42] | 4 |
| Lombardi |
Retrospective case control | Italy | 2003–2010 | 156 | 126 [8]/30 [20] | 0 |
| Miller |
Retrospective case control | USA | 2005–2011 | 156 | 110 [70]/46 [30] | Not available |
| Mir |
Retrospective case control | USA | 1993–2011 | 44 | 26 [59]/18 [41] | 27 |
| Porpiglia |
Retrospective case control | Italy | 2002–2008 | 43 | 25 [58]/18 [42] | Not available |
| Vanbrugghe |
Retrospective case control | France | 2002–2013 | 25 | 9 [36]/16 [64] | 0 |
OA, open laparotomic adrenalectomy; LA, laparoscopic adrenalectomy.
Advantages of minimally invasive surgery include decreased post-operative pain, shorter length of stay, quicker rehabilitation, improved cosmetic results, and more efficient use of healthcare expenditure; however, several concerns exist about the increased risk of tumor spillage, positive margins and earlier recurrence rates (29,38,68,72). Thus, technical feasibility of performing a minimally invasive removal is not the only criterion to deal with ACC.
It is evident that minimally invasive techniques may achieve gentle dissection of the adrenal mass from surrounding tissues, avoiding tumor rupture during direct manipulation only when specific skills have been acquired (29,38).
Since 2003 when the first International Adrenal Cancer Symposium was assembled to compare treatment regimens for ACC with the goal of defining standards of surgical care for this aggressive disease, several studies have compared the two surgical approaches (54).
In one of the last largest systematic review, Mpaili et al. (38) have compared the role of LA versus OA in ACC (ENSAT I–III) management, demonstrating the feasibility, safety, and potential benefits of the former.
All these observations have been also debated by an extensive meta-analysis made by Autorino et al. (73) that found LA favorable only in length of stay, when compared to the OA.
Finally, the recent National Comprehensive Cancer Network (NCCN) 2019 guidelines confirm that for suspected ACC with intermediate sizes (4–6 cm) laparoscopic surgery can be used or may be an exploring first approach with a rapid and planned conversion in case of evidence of local invasion (74).
LA appears to be equivalent to open method for localized/locally advanced primary ACC (ENSAT I–III), but data till now exposed and published are largely contradictory, lacking incontrovertible evidence of oncologic equivalence between the two approaches, in terms of complete resection rate, overall recurrence, disease free survival, and overall survival (38).
All the available studies focusing on this topic have several limitations. Most trials are observational and include a relative low number of patients (38,73). Nevertheless, the absence of randomized trials is recognized as a common drawback of clinical investigation for any surgical field above all regarding oncological outcomes (73). For this reason, the ESES/ENSAT collaborative group has given only suggestions and not recommendations that means that in this scientific set it is impossible to achieve a high grade of evidence recommendation and sufficient strength of recommendation (53).
Based on these findings, a more aggressive approach by open resection has been recommended for ACC lesions as well as for larger adrenal tumors of indeterminate malignant potential (54). However minimally invasive approach can be safely proposed in tertiary referral centers with surgical specific expertise, stringent preoperative selection, and early conversion to laparotomy in order to avoid any risk of tumor capsule rupture (63).
Patient factors, pre-operative considerations, tumor size and features and surgeon experience should dictate the right operative approach. Based on these considerations and the concept of the oncological radicality, recent comparative studies suggest that anterior transabdominal LA as well as posterior retroperitoneoscopic (PRA) approach might be equally safe, with similar operative times, blood loss, postoperative levels of patient discomfort, and recovery times, even if very large mass are challengingly removed by PRA because of the limited working space (16,75,76).
Minimally invasive adrenalectomy techniques: the robotic approach
The robotic surgical system has been recently adopted also for adrenalectomy. Horgan and Vanuno (77) reported the first robot-assisted laparoscopic adrenalectomy in 2001. Zafar and Abaza (78) in 2008 described the first report in the literature of robot-assisted LA for ACC. During the last 20 years more than 100 studies have evaluated the role of robotic adrenalectomy (RA) (78).
In most cases, RA and LA have shown similar outcomes (79). RA with transperitoneal approach might be more useful in case of large tumors and obese patients, decreasing mean operative time (80,81). However perioperative outcomes in obese patients are still controversial in comparison with the conventional laparoscopic approach (79,82).
The surgical robot system offers articulated instruments, three-dimensional (3D) vision, filtering of tremors, and a stable camera. Additionally, the surgeon can operate in a comfortable position. However, there are also disadvantages, such as high costs and no tactile feedback (82,83).
Increased costs associated with RA explain why LA is still more developed and frequently used. Actually, this minimally invasive technique is wider diffuse in the urology field than among endocrine surgeons probably because the former is more confident with the use of the robotic assisted surgery especially for retroperitoneal organs (79,80).
Although all the aforementioned efforts of the robotic surgery are well established for adrenalectomy in benign disease, a comparative analysis of surgical outcomes between RA and LA for adrenal malignancy remain scanty (84), because of the lack of long-term oncological outcome data (85).
Conclusions and future perspective
Several authors stressed the use of LA for smaller, localized tumors; however, the role of minimally invasive adrenalectomy for adrenal malignancies is still questionable, especially for ACC. A more permissive use of minimally invasive surgery in case of small adrenal metastases has been confirmed. Currently a more aggressive approach by open resection is recommended for ACC lesions as well as for larger adrenal tumors of indeterminate malignant potential. Well-designed randomized controlled studies are required to solve these controversies. A multidisciplinary evaluation with centralization of the management of adrenal malignancies in tertiary high-volume centers is mandatory to optimize patient outcomes (54).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giuseppe Cavallaro) for the series “Laparoscopic Endocrine Surgery” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2019.07.02). The series “Laparoscopic Endocrine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Gagner M, Lacroix A, Prinz RA, et al. Early experience with laparoscopic approach for adrenalectomy. Surgery 1993;114:1120-4; discussion 1124-5. [PubMed]
- Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma: clinical outcome at the end of the 20th century. Cancer 2001;92:1113-21. [Crossref] [PubMed]
- Icard P, Goudet P, Cyril Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg 2001;25:891-7. [Crossref] [PubMed]
- McCauley LR, Nguyen MM. Laparoscopic radical adrenalectomy for cancer: long-term outcomes. Curr Opin Urol 2008;18:134-8. [Crossref] [PubMed]
- Brix D, Allolio B, Fenske W, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncological outcome in 152 patients. Eur Urol 2010;58:609-15. [Crossref] [PubMed]
- Ushiyama T, Suzuki K, Kageyama S, et al. A case of Cushing’s syndrome due to adrenocortical carcinoma with recurrence 19 months after laparoscopic adrenalectomy. J Urol 1997;157:2239. [Crossref] [PubMed]
- Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007;356:601-10. [Crossref] [PubMed]
- . NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consens State Sci Statements 2002;19:1-25. [PubMed]
- Boland GW, Goldberg MA, Lee MJ, et al. Indeterminate adrenal mass in patients with cancer: evaluation at PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology 1995;194:131-4. [Crossref] [PubMed]
- Mayo-Smith WW, Lee MJ, McNicholas MM, et al. Characterization of adrenal masses (< 5 cm) by use of chemical shift MR imaging: observer performance versus quantitative measures. AJR Am J Roentgenol 1995;165:91-5. [Crossref] [PubMed]
- Corwin MT, Chalfant JS, Loehfelm TW, et al. Incidentally detected bilateral adrenal nodules in patients without cancer: is further workup necessary? AJR Am J Roentgenol 2018;210:780-4. [Crossref] [PubMed]
- Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 2010;7:754-73. [Crossref] [PubMed]
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab 2010;95:4106-13. [Crossref] [PubMed]
- Zeiger MA, Thompson GB, Duh QY, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: executive summary of recommendations. Endocr Pract 2009;15:450-3. [Crossref] [PubMed]
- Kiernan CM, Lee JE. Minimally Invasive Surgery for Primary and Metastatic Adrenal Malignancies. Surg Oncol Clin N Am 2019;28:309-26. [Crossref] [PubMed]
- Barzon L, Scaroni C, Sonino N, et al. Incidentally discovered adrenal tumors: endocrine and scintigraphic correlates. J Clin Endocrinol Metab 1998;83:55-62. [PubMed]
- Zhou J, Ye D, Wu M, et al. Bilateral adrenal tumor: causes and clinical features in eighteen cases. Int Urol Nephrol 2009;41:547-51. [Crossref] [PubMed]
- Carlson AL, Marney AM, Anderson SR, et al. Bilateral adrenal incidentalomas: a case report and review of diagnostic challenges. Case Rep Endocrinol 2013;2013:953052 [Crossref] [PubMed]
- Lee JE, Evans DB, Hickey RC, et al. Unknown primary cancer presenting as an adrenal mass: frequency and implications for diagnostic evaluation of adrenal incidentalomas. Surgery 1998;124:1115-22. [Crossref] [PubMed]
- Johnson PT, Horton KM, Fishman EK. Adrenal imaging with multidetector CT: evidence-based protocol optimization and interpretative practice. Radiographics 2009;29:1319-31. [Crossref] [PubMed]
- Elsayes KM, Emad-Eldin S, Morani AC, et al. Practical approach to adrenal imaging. Radiol Clin North Am 2017;55:279-301. [Crossref] [PubMed]
- Smith CD, Weber CJ, Amerson JR. Laparoscopic adrenalectomy: new gold standard. World J Surg 1999;23:389-96. [Crossref] [PubMed]
- Henry JF, Sebag F, Iacobone M, et al. Results of laparoscopic adrenalectomy for large and potentially malignant tumors. World J Surg 2002;26:1043-7. [Crossref] [PubMed]
- Moreno P, de la Quintana Basarrate A, Musholt TJ, et al. Adrenalectomy for solid tumor metastases: results of a multicenter European study. Surgery 2013;154:1215-22. [Crossref] [PubMed]
- Drake FT, Beninato T, Xiong MX, et al. Laparoscopic adrenalectomy for metastatic disease: Retrospective cohort with long-term, comprehensive follow-up. Surgery 2019;165:958-64. [Crossref] [PubMed]
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74-85. [Crossref] [PubMed]
- Ramsingh J, O'Dwyer P, Watson C. Survival outcomes following adrenalectomy for isolated metastases to the adrenal gland. Eur J Surg Oncol 2019;45:631-4. [Crossref] [PubMed]
- Puccini M, Panicucci E, Candalise V, et al. The role of laparoscopic resection of metastases to adrenal glands. Gland Surg 2017;6:350-4. [Crossref] [PubMed]
- Cho JW, Lee Y, Sung TY, et al. Factors related to improved clinical outcomes associated with adrenalectomy for metachronous adrenal metastases from solid primary carcinomas. Surg Oncol 2018;27:18-22. [Crossref] [PubMed]
- Marangos IP, Kazaryan AM, Rosseland AR, et al. Should we use laparscopic adrenalectomy for metastases? Scandinavian Multicenter Study. J Surg Oncol 2009;100:43-7. [Crossref] [PubMed]
- Twomey P, Montgomery C, Montet X, et al. Successful treatment of adrenal metastases from large-cell carcinoma of the lung. JAMA 1982;248:581-583. [Crossref] [PubMed]
- Romero Arenas MA, Sui D, Grubbs EG, et al. Adrenal metastectomy is safe in selected patients. World J Surg 2014;38:1336-42. [Crossref] [PubMed]
- Gryn A, Peyronnet B, Manunta A, et al. Patient selection for laparoscopic excision of adrenal metastases: A multicenter cohort study. Int J Surg 2015;24:75-80. [Crossref] [PubMed]
- Sebag F, Calzolari F, Harding J, et al. Isolated Adrenal Metastasis: The Role of Laparoscopic Surgery. World J Surg 2006;30:888-92. [Crossref] [PubMed]
- Strong VE, D’Angelica M, Tang L, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol 2007;14:3392-400. [Crossref] [PubMed]
- Vazquez BJ, Richards ML, Lohse CM, et al. Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg 2012;36:1400-5. [Crossref] [PubMed]
- Mpaili E, Moris D, Tsilimigras DI, et al. Laparoscopic Versus Open Adrenalectomy for Localized/Locally Advanced Primary Adrenocortical Carcinoma (ENSAT I-III) in Adults: Is Margin-Free Resection the Key Surgical Factor that Dictates Outcome? A Review of the Literature. J Laparoendosc Adv Surg Tech A 2018;28:408-14. [Crossref] [PubMed]
- Muth A, Persson F, Jansson S, et al. Prognostic factors for survival after surgery for adrenal metastasis. Eur J Surg Oncol 2010;36:699-704. [Crossref] [PubMed]
- Hirayama T, Fujita T, Koguchi D, et al. Laparoscopic adrenalectomy for metastatic adrenal tumor. Asian J Endosc Surg 2014;7:43-47. [Crossref] [PubMed]
- Sarela AI, Murphy I, Coit DG, et al. Metastasis to the adrenal gland: the emerging role of laparoscopic surgery. Ann Surg Oncol 2003;10:1191-6. [Crossref] [PubMed]
- Kebebew E, Siperstein AE, Clark OH, et al. Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal neoplasms. Arch Surg 2002;137:948-51. [Crossref] [PubMed]
- Taffurelli G, Ricci C, Casadei R, et al. Open adrenalectomy in the era of laparoscopic surgery: a review. Updates Surg 2017;69:135-43. [Crossref] [PubMed]
- Carnaille B. Adrenocortical carcinoma: which surgical approach? Langenbecks Arch Surg 2012;397:195-9. [Crossref] [PubMed]
- Lee CW, Salem AI, Schneider DF, et al. Minimally Invasive Resection of Adrenocortical Carcinoma: A Multi-Institutional Study of 201 Patients. J Gastrointest Surg 2017;21:352-62. [Crossref] [PubMed]
- Dickson PV, Kim L, Yen TWF, et al. Evaluation, Staging, and Surgical Managementfor Adrenocortical Carcinoma: An Update from the SSO Endocrine and Head and Neck Disease Site Working Group. Ann Surg Oncol 2018;25:3460-8. [Crossref] [PubMed]
- Ranvier GG, Inabnet WB 3rd. Surgical management of adrenocortical carcinoma. Endocrinol Metab Clin North Am 2015;44:435-52. [Crossref] [PubMed]
- Bellantone R, Ferrante A, Boscherini M, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery 1997;122:1212-8. [Crossref] [PubMed]
- Amin MB ES, Greene F, et al. AJCC Staging Manual. 8th ed. Springer International Publishing, 2017.
- Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 2009;115:243-50. [Crossref] [PubMed]
- Asare EA, Wang TS, Winchester DP, et al. A novel staging system for adrenocortical carcinoma better predicts survival in patients with stage I/II disease. Surgery 2014;156:1378-85; discussion 1385-76.
- Libé R, Borget I, Ronchi CL, et al. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol 2015;26:2119-25. [Crossref] [PubMed]
- Gaujoux S, Mihai R. joint working group of ESES and ENSAT. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg 2017;104:358-76. [Crossref] [PubMed]
- Winoker JS, Ahlborn DT, Omidele OO, et al. Minimally invasive adrenal surgery: virtue or vice? Future Oncol 2018;14:267-76. [Crossref] [PubMed]
- Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol 2013;169:891-9. [Crossref] [PubMed]
- Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol 2010;17:263-70. [Crossref] [PubMed]
- Porpiglia F, Miller BS, Manfredi M, et al. A debate on laparoscopic versus open adrenalectomy for adreno-cortical carcinoma. Horm Cancer 2011;2:372-7. [Crossref] [PubMed]
- Hermsen IG, Kerkhofs TM, den Butter G, et al. Surgery in adrenocortical carcinoma: Importance of national cooperation and centralized surgery. Surgery 2012;152:50-6. [Crossref] [PubMed]
- Kerkhofs TM, Verhoeven RH, Bonjer HJ, et al. Surgery for adrenocortical carcinoma in The Netherlands: analysis of the national cancer registry data. Eur J Endocrinol 2013;169:83-9. [Crossref] [PubMed]
- Fassnacht M, Johanssen S, Fenske W, et al. Improved survival in patients with stage II adrenocortical carcinoma followed up prospectively by specialized centers. J Clin Endocrinol Metab 2010;95:4925-32. [Crossref] [PubMed]
- Reibetanz J, Jurowich C, Erdogan I, et al. Impact of lymphadenectomy on the oncologic outcome of patients with adrenocortical carcinoma. Ann Surg 2012;255:363. [Crossref] [PubMed]
- Cooper AB, Habra MA, Grubbs EG, et al. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg Endosc 2013;27:4026-32. [Crossref] [PubMed]
- Donatini G, Caiazzo R, Do Cao C, et al. Long-term survival after adrenalectomy for stage I/II adrenocortical carcinoma (ACC): A retrospective comparative cohort study of laparoscopic versus open approach. Ann Surg Oncol 2014;21:284-91. [Crossref] [PubMed]
- Fosså A, Røsok BI, Kazaryan AM, et al. Laparoscopic versus open surgery in stage I-III adrenocortical carcinoma-A retrospective comparison of 32 patients. Acta Oncol 2013;52:1771-7. [Crossref] [PubMed]
- Gonzalez RJ, Shapiro S, Sarlis N, et al. Laparoscopic resection of adrenal cortical carcinoma: A cautionary note. Surgery 2005;138:1078-85. [Crossref] [PubMed]
- Leboulleux S, Deandreis D, Al Ghuzlan A, et al. Adreno-cortical carcinoma: Is the surgical approach a risk factor of peritoneal carcinomatosis? Eur J Endocrinol 2010;162:1147-53. [Crossref] [PubMed]
- Lodin M, Privitera A, Giannone G. Laparoscopic adrenalectomy (LA): Keys to success: Correct surgical indications, adequate preoperative preparation, surgical team experience. Surg Laparosc Endosc Percutan Tech 2007;17:392-5. [Crossref] [PubMed]
- Lombardi CP, Raffaelli M, De Crea C, et al. Role of laparoscopy in the management of adrenal malignancies. J Surg Oncol 2006;94:128-31. [Crossref] [PubMed]
- Miller BS, Gauger PG, Hammer GD, et al. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery 2012;152:1150-7. [Crossref] [PubMed]
- Mir MC, Klink JC, Guillotreau J, et al. Comparative outcomes of laparoscopic and open adrenalectomy for adrenocortical carcinoma: Single, high-volume center experience. Ann Surg Oncol 2013;20:1456-61. [Crossref] [PubMed]
- Vanbrugghe C, Lowery AJ, Golffier C, et al. Adrenocortical carcinoma surgery-surgical extent and approach. Langenbecks Arch Surg 2016;401:991-7. [Crossref] [PubMed]
- Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer 2005;12:667-680. [Crossref] [PubMed]
- Autorino R, Bove P, De Sio M, et al. Open versus laparoscopic adrenalectomy for adrenocortical carcinoma: a meta-analysis of surgical and oncological outcomes. Ann Surg Oncol 2016;23:1195-202. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Neuroendocrine And Adrenal Tumours Available online: http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Barczyński M, Konturek A, Nowak W. Randomized clinical trial of posterior retroperitoneoscopic adrenalectomy versus lateral transperitoneal laparoscopic adrenalectomy with a 5-year follow-up. Ann Surg 2014;260:740-7; discussion 747-8. [Crossref] [PubMed]
- Chai YJ, Yu HW, Song RY, et al. Lateral transperitoneal adrenalectomy versus posterior retroperitoneoscopic adrenalectomy for benign adrenal gland disease: randomized controlled trial at a single tertiary medical center. Ann Surg 2019;269:842-8. [Crossref] [PubMed]
- Horgan S, Vanuno D. Robotics in laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2001;11:415-9. [Crossref] [PubMed]
- Zafar SS, Abaza R. Robot-assisted laparoscopic adrenalectomy for adrenocortical carcinoma: initial report and review of the literature. J Endourol 2008;22:985-9. [Crossref] [PubMed]
- Nomine-Criqui C, Brunaud L, Germain A, et al. Robotic lateral transabdominal adrenalectomy. J Surg Oncol 2015;112:305-9. [Crossref] [PubMed]
- Brunaud L, Ayav A, Zarnegar R, et al. Prospective evaluation of 100 robotic-assisted adrenalectomies. Surgery 2008;144:995-1001; discussion 1001. [Crossref] [PubMed]
- Morelli L, Tartaglia D, Bronzoni J, et al. Robotic assisted versus pure laparoscopic surgery of the adrenal glands: a case– control study comparing surgical techniques. Langenbecks Arch Surg 2016;401:999-1006. [Crossref] [PubMed]
- Hupe MC, Imkamp F, Merseburger AS. Minimally invasive approaches to adrenal tumors: an up-to-date summary including patient position and port placement of laparoscopic, retroperitoneoscopic, robot-assisted, and single-site adrenalectomy. Curr Opin Urol 2017;27:56-61. [Crossref] [PubMed]
- Nomine-Criqui C, Germain A, Ayav A, et al. Robot-assisted adrenalectomy: indications and drawbacks. Updates Surg 2017;69:127-33. [Crossref] [PubMed]
- Mishra K, Maurice MJ, Bukavina L, et al. Comparative efficacy of laparoscopic versus robotic adrenalectomy for adrenal malignancy. Urology 2019;123:146-50. [Crossref] [PubMed]
- Quadri P, Esposito S, Coleoglou A, et al. Robotic adrenalectomy: are we expanding the indications of minimally invasive surgery? J Laparoendosc Adv Surg Tech A 2019;29:19-23. [Crossref] [PubMed]
Cite this article as: Schiavone D, Torresan F, Negro S, Belluzzi A, Iacobone M. Adrenal malignancy: still a contraindication for laparoscopy? Laparosc Surg 2019;3:30.