
Reply to letter: “Laparoscopic liver resection in cirrhosis: the challenge of the posterosuperior segments”
We thank Dr. August and Dr. Visser for their interest and comments regarding our study on the feasibility of minimal-invasive procedures to treat lesions in the posterosuperior segments of the liver (1). As appropriately pointed out, the inclusion of patients undergoing major hepatectomy for a lesion in the posterosuperior segments limits the ability to evaluate the technical difficulties one is likely to encounter when performing isolated posterosuperior segmentectomy. While the main goal of our study was to assess whether lesions in the posterosuperior segments can be addressed through minimal invasive techniques rather than whether sectionectomy of the posterosuperior segments is feasible, we address this point through a sub-analysis of local resections, where outcomes were similar between cirrhotic and non-cirrhotic patients as well (1). We agree however, that the difficulty of a particular resection is best characterized through the intended resection type rather than the location of the lesion. In keeping with this, the established scores such as the IWATE criteria that guide liver surgeons in appraising the difficulty of a laparoscopic procedure might benefit from modification that places more weight on the segments to be resected than the distinct location of the lesion (2,3).
Despite considerable advantages in the field of laparoscopic hepato-pancreatico-biliary (HPB) surgery, indication criteria remain obscure. Certainly, over the last few years we have seen a marked shift towards laparoscopic surgical oncology (4). Our findings in this study are in accordance with the more general observation that patients with liver cirrhosis seem to benefit disproportionately from the application of minimal invasive techniques as these are able to elicit faster recovery while achieving similar safety results compared to non-cirrhotic patients (5). Similar to the worldwide trend we can report a shift in the field of laparoscopic liver surgery from resections for benign lesions to complex surgical oncology. Indeed, tumor characteristics such as size and the adjacency to the hepatic veins are in the hand of an experienced laparoscopic-adept liver surgeon no contraindication towards minimal-invasive techniques. Since 2017, the proportion of cases performed laparoscopically has further increased at our center. Aside from cases requiring hepaticojejunostomy or vascular reconstruction or those with extensive surgical history in the upper abdomen, most hepatocellular carcinoma (HCC) cases in cirrhosis are now performed minimally-invasive with an increasing proportion of those patients undergoing robotic resection. Almost all patients in our cohort had well preserved liver function (Child-Pugh A) and a model for end-stage liver disease (MELD) score of up to 10 (1). As most patients with cirrhosis undergoing resection are treated for HCC Barcelona clinic liver cancer (BCLC) 0-A, curative alternatives can be applied in case the patient is not an ideal candidate for resection. Indication for resection in patients with MELD-scores above 10 was given on a case-by-case basis after discussion in an interdisciplinary tumor board.
Elsewhere we have reported outcomes of all patients undergoing minimal-invasive liver surgery as compared to patients undergoing laparotomy applying propensity score matching (6). Consistently, minimal-invasive procedures were associated with shorter hospitalization and less morbidity in HCC patients independent of the distinct location of the lesion. However, indication for the distinct surgical technique is selected at the operating surgeon’s discretion and subsequent analysis may entail a selection bias.
As described in our report, we have in recent years increasingly applied the diamond technique to perform resection (7). Herein, a 12 mm trocar is placed through the umbilicus for the camera. Further 12 mm trocars are placed in two direct lines: first, between umbilicus and xiphoid, and second, on a horizontal line between umbilicus and the right lateral costal arch. In order to facilitate the diamond technique, it is pivotal that two 12 mm trocars are placed along each of these 2 lines. This will allow for superior maneuverability when defining the transection plains and performing resection. Prior to resection, a square-shaped transection area is outlined. Selecting the appropriate tool at one’s disposal to perform resection in fibrotic parenchyma is critical. During the learning curve we applied different shears (Thunderbeat®, Olympus or Harmonic Ace®, Ethicon or UltraCision®, Ethicon Inc. Somerville, NJ, USA) as well as CUSA or vascular staplers (Echelon Flex®, Ethicon, Somerville, NJ, USA). Recently, however, we most commonly apply waterjet (ERBEJET®, Tübingen, Germany) with 40 mbar. In order to decrease hemorrhage, we perform need-based intermittent pringle maneuver repeatedly for 15 minutes followed by 5 minutes of unimpeded perfusion. Based on our experience as well as other reports, this has no implication for liver function as neither postoperative transaminase levels nor overall complications are increased independent of how many times pringle maneuver was performed (8).
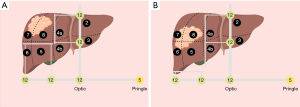
Ultimately, distinguishing between isolated resections of the posterosuperior segments and more extended resections for lesions located in those segments has an impact on the difficulty of the resection. However, as depicted in Figure 1. there is no difference in trocar placement when performing bisegmentectomy for a posterosuperior lesion and a right hemihepatectomy. The importance of performing diligent mobilization of the right liver even when only local resection is performed can hardly be overstated. Cleaving the falciform, triangular and right coronary ligament provides exposure particular to the superior liver segments as the anterior liver surface can be tilted easier and the surgeon is able to incise the liver at a more obtuse angle, which in turn reduces the likelihood of incising tumor tissue.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Laparoscopic Surgery. The article did not undergo external peer review.
Conflicts of Interest: The authors have direct or indirect conflicts of interests relevant to the article as follows: Johnson & Johnson Medical GmbH, ERBE Elektromedizin GmbH, Intuitive Inc.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haber PK, Wabitsch S, Krenzien F, et al. Laparoscopic liver surgery in cirrhosis—addressing lesions in posterosuperior segments. Surg Oncol 2019;28:140-4. [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Krenzien F, Wabitsch S, Haber P, et al. Validity of the Iwate criteria for patients with hepatocellular carcinoma undergoing minimally invasive liver resection. J Hepatobiliary Pancreat Sci 2018;25:403-11. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Yoon YI, Kim KH, Cho HD, et al. Long-term perioperative outcomes of pure laparoscopic liver resection versus open liver resection for hepatocellular carcinoma: a retrospective study. Surg Endosc 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Andreou A, Struecker B, Raschzok N, et al. Minimal-invasive versus open hepatectomy for hepatocellular carcinoma: comparison of postoperative outcomes and long-term survivals using propensity score matching analysis. Surg Oncol 2018;27:751-8. [Crossref] [PubMed]
- Cipriani F, Shelat VG, Rawashdeh M, et al. Laparoscopic parenchymal-sparing resections for nonperipheral liver lesions, the diamond technique: technical aspects, clinical outcomes, and oncologic efficiency. J Am Coll Surg 2015;221:265-72. [Crossref] [PubMed]
- Dua MM, Worhunsky DJ, Hwa K, et al. Extracorporeal pringle for laparoscopic liver resection. Surg Endosc 2015;29:1348-55. [Crossref] [PubMed]
Cite this article as: Haber PK, Kästner A, Pratschke J, Schmelzle M. Reply to letter: “Laparoscopic liver resection in cirrhosis: the challenge of the posterosuperior segments”. Laparosc Surg 2019;3:43.


