
Bilateral adrenalectomy—simultaneous or delayed?
Background
After the first reports of laparoscopic adrenalectomy were published in 1992 by Higashihara in Japan (1) and by Gagner in Canada (2) the technique has become widely adopted in many surgical centers. A similar but slower uptake has been noticed for the retroperitoneoscopic approach popularized by Martin Walz (3). As with all other minimally invasive operations, a shorter in hospital stay and lower perioperative morbidity had a significant impact on the feasibility of this operation for a larger number of complex patients.
Open bilateral adrenalectomy was first reported in early 1950s as a treatment for essential hypertension (4) and later used in women with advanced breast cancer (5). As those indications are obsolete, in recent years the indication for minimally invasive bilateral adrenalectomy remains very limited.
The 2017 report of the national audit run by the British Association of Endocrine and Thyroid Surgeons showed that only 90 bilateral adrenalectomies were performed in a large cohort of 2,073 patients who underwent adrenal surgery (6) therefore the experience with this operation remains extremely limited.
The aim of this paper is to define the indications for bilateral adrenalectomy, discuss its technical feasibility and limitations and comment on the practical issues of service provision.
Who can be considered for bilateral adrenalectomy?
There are three separate indications for bilateral adrenalectomy in the same index operation: (I) bilateral phaeochromocytomas; (II) ACTH-independent bilateral adrenal hyperplasia; (III) ACTH-dependent bilateral adrenal hyperplasia after failed pituitary surgery or in the presence of ectopic ACTH secretion.
Bilateral PHAEOs
Bilateral PHAEOs occur in patients with genetic syndromes caused by mutations in genes known to affect tumorigenesis in the chromaffin adrenal cells. Even if such patients present with unilateral tumors but are at risk of developing a contralateral adrenal tumor (or an extraadrenal tumor) later in life. Only a minority of these patients present initially with bilateral disease.
Multiple endocrine neoplasia type II
Multiple endocrine neoplasia type II is an inherited dominant condition caused by mutations in the RET gene. All patients with MEN2A should be presumed to have a significant life-time risk of developing bilateral PHAEOs as these occur in over 50% of patients. Malignancy is rare in MEN2 patients, with only two malignant PHAEOs identified in a large cohort of 563 patients (7). Bilateral subtotal ADX is therefore desirable and effective in these patients.
A large study from University Medical Centre Utrecht analysed their experience between 1959–2010 and found that MEN2 patients underwent 22 bilateral total, 2 bilateral subtotal, 30 unilateral total and 7 unilateral subtotal adrenalectomies. After a follow-up of 13±10 years, 13 patients developed contralateral recurrence after a median of 10 years (8).
In a large pan-European study of 345 patients with MEN2B syndrome caused by Met918Thr mutation, bilateral synchronous PHAEOs were the initial diagnosis in 79 patients (half of the patients who were observed to have developed a PHAEO). Of 26 patients who had bilateral subtotal ADX, normal adrenocortical function was maintained in 62% (9).
Von Hippel Lindau syndrome
Von Hippel Lindau syndrome is a genetic syndrome caused by mutations in the VHL gene. Up to a third of patients will develop PHAEOs, of which 40–50% are bilateral. Because malignancy rate is low, subtotal ADX is a safe option in both adults and children with VHL (10,11).
Neurofibromatosis type 1 (NF1)
PHAEOs used to be deemed as rare (5%) but recent series reported a 15% incidence. Two thirds of patients are asymptomatic and remain undetected without routine screening. Bilateral disease is expected in 11–27%. Those who develop PHAEOs are also likely to have bilateral tumors.
Currently it is believed that up to 30% of PHAEOs are due to genetic mutations in up to 10 genes. For some of these mutations there is minimal knowledge whether phenotype-genotype correlation can influence clinical decisions making at the time of their original presentation. This topic has been discussed extensively in a recent review (12).
ACTH-independent bilateral adrenal hyperplasia (Cushing syndrome with bilateral adrenal disease)
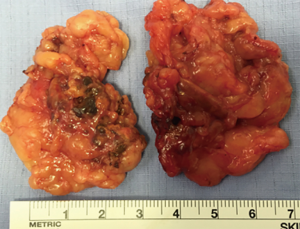
The largest report series from Essen, Germany described 42 patients with ACTH-independent hypercortisolism and bilateral adrenal disease operated during 2004-2016. 16 patients with asymmetric enlargement had unilateral ADX and 26 patients had bilateral ADX (subtotal resections (n=9), ADX + contralateral subtotal ADX (n=14), bilateral total ADX (n=3) (13). This rare clinical scenario needs individualised decision based on agreement between medical team, surgical team and patient. In our clinical practice we encountered a single patient with bilateral pigmented macronodular hyperplasia for which a bilateral adrenalectomy was performed retroperitoneoscopically (Figure 1).
ACTH-dependent bilateral adrenal hyperplasia
Failed pituitary surgery for Cushing disease is the most common scenario but recent improvements in the efficacy of pituitary surgery have reduced significantly the number of such patients. Bilateral ADX is used as a ‘salvage treatment’ in those with severe signs/symptoms of hypercortisolism after failure of medical therapy.
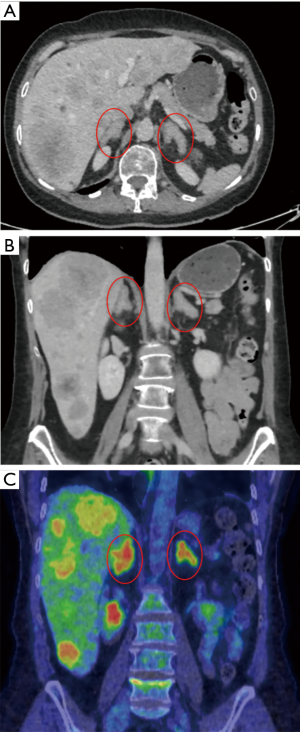
Ectopic secretion of ACTH from neuroendocrine tumors can lead to bilateral adrenal hyperplasia. When treatment of the primary tumor is not feasible (e.g., unknown primary site) or in the presence of extensive metastatic disease non-responding to other oncological treatments it becomes beneficial to proceed with bilateral ADX with the aim of reducing the morbidity of uncontrolled hypercortisolism. One such case is presented in Figure 2.
Metastatic disease from non-adrenal tumors
Even though unilateral ADX is increasingly recommended for patients with oligometastatic tumors (14), the oncological/clinical benefits of bilateral ADX for bilateral adrenal metastases are yet to be demonstrated and have to be considered on a case-by-case discussion.
Feasibility of bilateral adrenalectomy
If clinically beneficial, it is possible to offer bilateral ADX either as a laparoscopic or a retroperitoneoscopic operation. The choice of technique is influenced predominantly by the expertise available in each medical center. The laparoscopic approach has the disadvantage of having to change the position of the patient on the operating table before proceeding with contralateral surgery. This is not a problem during retroperitoneoscopic surgery, a technique that brings the additional advantage of being possible to have two teams operating simultaneously on each side (hence reducing the length of the procedure)—an option only in centers where there are two surgeons with appropriate skills.
Total or subtotal adrenalectomy?
As mentioned above, subtotal ADX is a reasonable option in a subgroup of patients with familial PHAEOs. Feasibility of subtotal ADX relies on avoidance of excessive gland dissection aiming to maintain peripheral venous and arterial branches feeding the remnant adrenal. The central vein can be divided if necessary.
Retroperitoneoscopic approach is more ‘forgiving’ for subtotal ADX as the bleeding from the adrenal remnant is easier to control.
Assessing adequate residual volume is unpredictable. Remnants of about one-third of the total original gland size predicted adequate function on the basis of synacthen stimulation. A remnant size as small as 15% of that of the normal gland would allow exogenous steroid independence, although subclinical adrenal hypofunction, on the basis of an inadequate response to synacthen (15).
Simultaneous or delayed bilateral adrenalectomy?
There is no need for delayed surgery when adequate surgical and anesthetic expertise exists. The operation can be achieved with minimal blood loss, in relatively short operating time and without significant morbidity. In our published report we described 23 cases operated between 2005–2016 (16). Median length of hospital stay was 5 days and postoperative complications (Clavien-Dindo classification) included one chest infection (level 2), one postoperative hemorrhage and two chest drains for pneumothorax (level 3), two postoperative cardiac arrests (level 4) and one late cancer death from complications related to uncontrolled hypercortisolism (level 5).
Long-term morbidity after total bilateral adrenalectomy
Patients need life-long steroid replacement, with a risk of 8.3 Addisonian crises per 100 patients-years (17). Furthermore excessive corticosteroid replacement is associated with premature osteoporosis, diabetes and hypertension. For these reasons, when deemed to be safe/feasible, bilateral subtotal ADX provides better long-term results.
The alternative of heterotopic autotransplantation has a low success rate of 0–36% when small fragments transplanted into small pockets in the muscle or in omentum or subcutaneous fat followed by ACTH injections to promote growth (18).
Service provision
The current provision of adrenal surgery is in critical need for radical change. Reports from several countries demonstrated that the operation continues to be performed in centers with minimal annual workload. In an analysis of hospital admissions of 795 adrenalectomies in the United Kingdom for the year 2013–2014, 198 surgeons performed <6 cases/year (median of 1case/year!) and only 36 surgeons performed >6 cases/year (19). In France 9820 adrenalectomies were performed between 2012–2017, with 280 centers performing <5 adrenalectomies during the 6-year period of the study. Only 26 centers performed half of the national instances of adrenalectomy (20). In the United States, 50% of patients who underwent adrenal surgery 1998–2000 were operated by surgeons who performed <2 operations (21) and a decade later the situation changed very little as the upper quartile of the distribution of workload showed that high-volume surgeons were defined as those performing 4 or more cases /year (22).
It has been demonstrated that low-volume surgeons have higher complication rates, longer length of stay and provide more expensive care [for a review see (23)]. There is therefore a clear need to centralize adrenal surgery and stratify cases that can be managed locally by surgeons performing over 6 cases/year and refer high risk patients (e.g., adrenocortical cancers and PHAEOs or patients with significant comorbidities) towards regional centers were the workload would be expected to be at least 12 cases/year and all patients should be discussed via a multidisciplinary approach. These issues were discussed extensively during the 2019 meeting of the European Society of Endocrine Surgeons (23) with the expectation that in coming years the service provision will be reorganized through a process involving professional bodies, patients’ representatives and political initiatives.
As bilateral adrenalectomy is indicated in patients that raise the most significant challenges, their care should be not allowed in medical institutions with minimal/no experience in dealing with complex adrenal cases.
Summary
- Indications for bilateral ADX should be discussed in a multidisciplinary environment including a surgeon with significant personal experience in adrenal surgery.
- When indicated, bilateral adrenalectomy can be done simultaneously as a laparoscopic or retroperitoneoscopic procedure.
- Understanding the significance of particular genetic mutations in patients with familial PHAEOs is paramount before offering bilateral (subtotal) adrenalectomy.
- Subtotal bilateral adrenalectomy provides a reasonable chance (but not a guarantee) of avoiding steroid dependence.
- There is a need to centralize adrenal surgery in several centers in each country.
Acknowledgments
Some of these data was presented at ECE2019 (
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giuseppe Cavallaro) for the series “Laparoscopic Endocrine Surgery” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2019.06.03). The series “Laparoscopic Endocrine Surgery” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Higashihara E, Tanaka Y, Horie S, et al. A case report of laparoscopic adrenalectomy. Nihon Hinyokika Gakkai Zasshi 1992;83:1130-3. [Crossref] [PubMed]
- Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Walz MK, Peitgen K, Walz MV, et al. Posterior retroperitoneoscopic adrenalectomy: lessons learned within five years. World Journal of Surgery 2001;25:728-34. [Crossref] [PubMed]
- Decourcy JL. Subtotal bilateral adrenalectomy for hyperadrenalism (essential hypertension). Ann Surg 1934;100:310-8. [Crossref] [PubMed]
- Devitt JE, Hardwick JM. Role of bilateral adrenalectomy (and oophorectomy) in the management of patients with metastatic breast cancer. Am J Surg 1979;137:629-33. [Crossref] [PubMed]
- BAETS National audit report. Available online: https://www.baets.org.uk/wp-content/uploads/BAETS-Audit-National-Report-2017.pdf, accessed 6th June2019.
- Castinetti F, Qi XP, Walz MK, et al. Outcomes of adrenal-sparing surgery or total adrenalectomy in phaeochromocytoma associated with multiple endocrine neoplasia type 2: an international retrospective population-based study. Lancet Oncol 2014;15:648-55. [Crossref] [PubMed]
- Scholten A, Valk GD, Ulfman D, et al. Unilateral subtotal adrenalectomy for pheochromocytoma in multiple endocrine neoplasia type 2 patients: a feasible surgical strategy. Ann Surg 2011;254:1022-7. [Crossref] [PubMed]
- Castinetti F, Waguespack SG, Machens A, et al. Natural history, treatment, and long-term follow up of patients with multiple endocrine neoplasia type 2B: an international, multicentre, retrospective study. Lancet Diabetes Endocrinol 2019;7:213-20. [Crossref] [PubMed]
- Benhammou JN, Boris RS, Pacak K, et al. Functional and oncologic outcomes of partial adrenalectomy for pheochromocytoma in patients with von Hippel-Lindau syndrome after at least 5 years of follow-up. J Urol 2010;184:1855-9. [Crossref] [PubMed]
- Volkin D, Yerram N, Ahmed F, et al. Partial adrenalectomy minimizes the need for long-term hormone replacement in pediatric patients with pheochromocytoma and von Hippel-Lindau syndrome. J Pediatr Surg 2012;47:2077-82. [Crossref] [PubMed]
- Rossitti HM, Söderkvist P, Gimm O. Extent of surgery for phaeochromocytomas in the genomic era. Br J Surg 2018;105:e84-e98. [Crossref] [PubMed]
- Lowery AJ, Seeliger B, Alesina PF, et al. Posterior retroperitoneoscopic adrenal surgery for clinical and subclinical Cushing's syndrome in patients with bilateral adrenal disease. Langenbecks Arch Surg 2017;402:775-85. [Crossref] [PubMed]
- Russo AE, Untch BR, Kris MG, et al. Adrenal Metastasectomy in the Presence and Absence of Extraadrenal Metastatic Disease. Ann Surg 2018; [Epub ahead of print]. [PubMed]
- Brauckhoff M, Gimm O, Thanh PN, et al. Critical size of residual adrenal tissue and recovery from impaired early postoperative adrenocortical function after subtotal bilateral adrenalectomy. Surgery 2003;134:1020-7. [Crossref] [PubMed]
- Maccora D, Walls GV, Sadler GP, Mihai R. Bilateral adrenalectomy: a review of 10 years' experience. Ann R Coll Surg Engl 2017;99:119-22. [Crossref] [PubMed]
- Hahner S, Hemmelmann N, Quinkler M, et al. Timelines in the management of adrenal crisis - targets, limits and reality. Clin Endocrinol (Oxf) 2015;82:497-502. [Crossref] [PubMed]
- Iacobone M, Citton M, Viel G, et al. Surgical approaches in hereditary endocrine tumors. Updates Surg 2017;69:181-91. [Crossref] [PubMed]
- Palazzo F, Dickinson A, Phillips B, et al. Adrenal surgery in England: better outcomes in high-volume practices. Clin Endocrinol (Oxf) 2016;85:17-20. [Crossref] [PubMed]
- Caiazzo R, Marciniak C, Lenne X, et al. Adrenalectomy Risk Score: an original pre-operative surgical scoring system to reduce mortality and morbidity after adrenalectomy. Ann Surg 2019; [Crossref]
- Saunders BD, Wainess RM, Dimick JB, et al. Who performs endocrine operations in the United States? Surgery 2003;134:924-31; discussion 931. [Crossref] [PubMed]
- Kuo JH, Pasieka JL, Parrack KM, et al. Endocrine surgery in present-day academia. Surgery 2014;156:1461-9. [Crossref] [PubMed]
- Mihai R, Donatini G, Vidal O, et al. Volume-outcome correlation in adrenal surgery - an ESES consensus statement. Langenbecks Arch Surg 2019; [Crossref]
Cite this article as: Mihai R. Bilateral adrenalectomy—simultaneous or delayed? Laparosc Surg 2019;3:47.


