
Various techniques for bile duct division in pure laparoscopic donor hepatectomy
Introduction
Laparoscopic liver surgery (LLS) has been accepted as a tool of the management of several kind of liver diseases in the world. It has advantage of short hospital stay, early recovery and wound cosmetic aspect. The initial period, there was many trial to make safe and feasible technique (1,2). Even the expert panel found that laparoscopic adult living donor hepatectomy is in the development phase with unclear benefit-risk ratio and long-term outcomes of donors and recipients (3). However, the indications for LLS has expanded recently from minor liver resect to major liver resections for various liver tumors with feasible short- and long-term outcomes (4-6). And indication of LLS now expanded to living donor hepatectomy. Many centers have reported their feasible and excellent outcome of pure laparoscopic donor hepatectomy (7-11). However, biliary complications after liver transplantation are a concern for both donors and recipients. Appropriate bile duct division using laparoscopic instruments should be emphasized in this aspect. There were many efforts to ensure accurate bile duct division. For example, Primovist MRI can be used to confirm the bile duct anatomy before surgery, or intraoperative cholangiography can be used to accurately perform bile duct division (12,13). Recently, ICG cholangiography has been reported to help safely perform bile duct division during surgery (14). However, there are still some differences in how to handle bile duct stumps. We describe different methods and chronical changes of bile duct division methods in pure laparoscopic donor hepatectomy. This report aims to describe our procedure for safe pure laparoscopic adult living donor hepatectomy with several techniques of bile duct division.
Methods
Between January 2016 and December 2018, we reviewed the 92 cases of adult living-donors who underwent pure laparoscopic donor hepatectomy by a single surgeon (Lee K.W.) at Seoul National University Hospital, Seoul National University, Korea. We described the technique of bile duct division in three different ways. This study was approved by the Institutional Review Board (IRB) at Seoul National University Hospital (SNUH) number “1812-062-993” which waived the need for informed consent.
Surgical technique
The surgical technique had been described before (Hong et al., 2018) (15). Here, we describe a single surgeon (Lee K.W.) technique, in short, emphasizing important technical tips for pure laparoscopic adult living donor hepatectomy for donors focused on bile duct division (Figure 1).
Donor position and Port placement
The donor was placed in lithotomy and reverse Trendelenburg position with right side upward tilt. The surgeon stands between the donor’s legs, assistant and scopist stand on the donor’s left side. CO2 pneumoperitoneum was maintained at 12 mmHg, and five port system was used.
Liver parenchymal transection
The superficial layer of the liver parenchyma is divided using energy device (THUNDERBEAT®; Olympus Medical Systems Corp., Tokyo, Japan). The deep portion of the parenchyma is transected by cavitron ultrasonic suction aspirator (CUSA®; Valleylab, Inc., Boulder, CO, USA). Hepatic veins draining segments V and VIII were clamped by Hem-O-Lok and divided to be reconstructed on bench surgery. After transecting the main part of the parenchyma, the hilar plate is approached. Real-time ICG near-infrared fluorescence cholangiography is used to identify bifurcation of the right and left hepatic ducts
Identification of Bile duct with ICG cholangiography
All donors were assessed preoperatively for a history of iodine allergy and anaphylactic reactions to any other drugs. ICG (0.05 mg/kg) was injected intravenously 60 minutes before the dissection of the hilar plate. After comparing real-time ICG fluorescence cholangiography and preoperative delayed phase of Primovist MRI image, the bifurcation of the right and left hepatic ducts were carefully dissected. When the right or left hepatic duct was well visualized, the appropriate resection point of the bile ducts was determined. After rechecking the patency of the remnant side of the bile ducts and the common bile duct (CBD), we resected the bile duct along the marked line.
Bile duct division
Suture
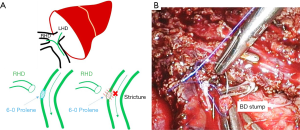
This method is mainly used in open donor hepatectomy. After identifying the right hepatic duct, applying the tagging suture between right and left bifurcation site with 6-0 Prolene. Pull downward the tagging suture by assistant then we resected right bile duct. After resection, the stump was closed with continuous suture using 6-0 Prolene. At this time, lifting the initial point of the suture by using the grasp of the assistant. This can prevent the bile duct stricture of the remnant left hepatic duct (Figure 1).
Metal clip
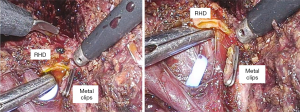
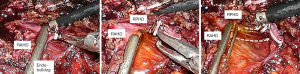
After checking the position of the right bile duct, tunnel through the Y-shape using the dissector. At this time, lower the power of the electrocautery to prevent damage to the biliary tract that may occur elsewhere. After tunneling, isolate the right bile duct with a dissector and ligation one 10mm metal clip. Then we rechecked the ICG cholangiography to determine if the distance to CBD is appropriate. Then we also applied the 10mm clip in the same way, and resect the right bile duct. If we performed bile duct division to the case of multiple bile duct opening, this method can be applied repeatedly. It is easy and quick to apply and has a merit that it is easy to apply and can be applied to the case of a short length of right CBD because the shape is thin. (Figure 2)
Hem-O-Lok
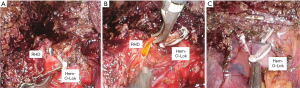
As we mentioned the way to use the metal clip described above, the right bile duct is separated and then Hem-o-Lok can be applied. If there is not enough space for two Hem-O-Loks, you can apply another 10 mm metal clip on the top. It is better to apply to a thick or wide bile duct than the metal clip because the metal clip sometimes slipped from remnant thick bile duct stump. And it prevents the massive dissection around bile duct that could because of bile duct ischemia (Figure 3).
Remnant Glisson tissue ligation
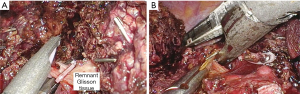
Remnant Glisson tissue should be ligated for preventing bile leakage from small branch of caudate duct. It can be performed by Hem-O-Lok or Endostapler. Hem-O-Lok is suitable usual case, Endostapler is suitable for wide Glisson tissue division. When we used the stapler for this procedure, we need more space between portal vein and bile ducts. Especially, if the right posterior duct cross over the portal vein, this procedure would be dangerous. Therefore, we should be careful for preventing injury to right post duct during stapling (Figure 4).
Results
In this study, we present a single surgeon’s tip of various bile duct division in pure laparoscopic adult living-donor hepatectomy. We used intraoperative ICG cholangiography for checking bile duct anatomy in every case. The biliary stricture occurred only 1 case of the suture method group. There was no stricture or leakage in metal clips and Hem-o-Lok clip groups. We described the merit and drawbacks of each method in Table 1.
Table 1
| Suture | Metal Clips | Hem-O-Lok Clips | |
|---|---|---|---|
| Merits | • Safe | • Good control of small sized bile duct (separated 2BD) | • Removable and re-adjustable |
| • Similar to open approach | • Safe and precise | • Easily applied in limited space between RPV branches | |
| • No concerns about slippage of closing material | • Requires narrow space | ||
| • Easy to apply additional clips | |||
| Drawbacks | • Time consuming | • High chance of slippage | • Possible slippage and subsequent bleeding |
| • Requires meticulous suturing skills | • Requires more wider space than metal clips | ||
| • Possible slippage and subsequent bleeding during suturing | • Huge remnant material |
Operative outcomes
No open conversions occurred in this series. The mean operative time of donors was 227.6±68.46 minutes and the mean estimated blood loss was 241.8±177.3 cc. None of the donors in this series received intra- or postoperative blood transfusion. All donors started ambulation on the 1 day after the operation. Sips of water was started on the first post-operative day. Most donors had no biliary complication. Only one donor (1.1%) had a biliary stricture in the case of the suture closure method. This donor developed prolonged cholestasis requiring re-operation (grade III morbidity, according to Clavien-Dindo classification) (16). It was related to suture material for remnant bile duct stump closure. After re-operation, it is not necessary to do additional treatment for this donor. We changed our policy of the bile duct division method after this event. There was no re-hospitalization due to biliary complications that were required intervention during the follow-up period of the donors in this series. In the recipients, there were 7 cases (7.6%) of biliary complication that was required intervention. There was no mortality related to bile duct complications in these recipients.
Discussions
Bile duct division is the most important step for reducing biliary complications after liver transplantation in both donors and recipients. Therefore, it is important to know the exact structure of the biliary tract before and during surgery. Some centers do not offer laparoscopic donor surgery in consideration of donor safety in the donor with bile duct anatomic variations (7,17). An intraoperative cholangiogram also has been used to identify the anatomy of the biliary tract during surgery for safe bile duct division. Recently, preoperative Primovist MRI has been used to identify the anatomy of the biliary tract in a non-invasive manner. Despite these efforts, bile duct division is still an important challenge to overcome in pure laparoscopic donor surgery. In the case of laparoscopic donor hepatectomy, it is not easy to confirm the bile duct during surgery as compared to open donor hepatectomy, and if it is not safely ligated, the donor may have problems such as leakage of the bile and biliary stricture. Therefore, learning the methods for safe bile duct division in pure laparoscopic donor hepatectomy is a key factor for donor safety and good surgical outcomes in the center that want to set up pure laparoscopic donor hepatectomy.
SNUH's initial principle of laparoscopic donor hepatectomy is the same way with open donor surgery should be applied in laparoscopic donor surgery. Initially, the suture method was used like an open donor surgery. There was no big problem in initial cases, but the laparoscopy suture method is relatively difficult compared to the open surgery and it is relatively unfavorable for implantation of graft bile duct because it must contain enough stump. The most significant disadvantage was that stenosis of the left bile duct could be occurred in some case and that might be related to difficulty of laparoscopic suture to small bile ducts in the case of multiple bile duct donors. Recently, we changed the primary method with a metal clip or hem-O-Lok. But, if there were bile spillage from bile duct stump in several cases or if it is difficult to apply a large material such as a metal clip or hem-O-Lok due to narrow space, the suture can also be a good alternative.
Since ICG cholangiography has been used, accurate anatomy of the biliary tract can be identified, making it easier to use metal clips or hem-O-Lok (17,18). It is easy to encircle the bile duct, and depending on the size, the biliary duct can be sufficiently ligated with only a metal clip. Even in the case with bile duct variation, we can perform safely based on ICG image (Figure 5) (19).
However, similar with case of cholecystectomy, the metal clip sometimes slippages when ligation of thick ducts. In these cases, hem-O-Lok could be a good alternative. However, because hem-O-Lok is relatively thick and large, it is important to make an appropriate selection according to the size and thickness of the bile ducts. Table 1 describes the advantages and disadvantages of each method. The limitation of this study is not a comparative study of each method because we chose each method by considering operative filed, bile duct thickness, and number of duct opening. Therefore, each group has different demographics and not homogenous. Currently, the method will be updated chronically using mainly hem-O-Lok. Therefore, comparing the results of these three methods is not appropriate considering factors such as the learning curve. As mentioned above, the main purpose of this study is to show that each method can be applied in various cases depending on the advantages and disadvantages of each method and the overall results of applying the three methods are feasible. We wanted to focus on the technical issues about method of bile duct division and each merit and drawbacks. There have been recent reports of laparoscopic donor studies and they are known to be feasible for their stability and complications. However, few studies described detail about the bile duct division methods. Because almost studies focused primarily on outcomes rather than detailed descriptions of surgical methods, there is a lack of adequate guidance and useful information for the surgeon in centers that are preparing for laparoscopic donor surgery. SNUH currently achieved more than 350 cases of pure laparoscopic donor hepatectomy. Based on these experiences, merit and drawbacks about various methods of bile duct division are described in this article. It will be a good guide to future laparoscopic donor surgeons.
In conclusion, laparoscopic adult living donor hepatectomy is a technically challenging procedure requiring many advanced laparoscopic techniques. Various bile duct division technique had several merits and drawbacks. We should choose the appropriate method for safe pure laparoscopic donor hepatectomy. The current series will be a step towards further expansion of the laparoscopic adult living donor hepatectomy in the world by giving them information about the detailed surgical procedure of bile duct division.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Laparoscopic Surgery for the series “Pure Laparoscopic Donor Hepatectomy”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2020.02.06). The series “Pure Laparoscopic Donor Hepatectomy” was commissioned by the editorial office without any funding or sponsorship. KWL and JML served as the unpaid Guest Editors of the series. KWL also serves as an unpaid editorial board member of Laparoscopic Surgery from Jan 2019 to Dec 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board at Seoul National University Hospital (SNUH) [number 1812-062-993] which waived the need for informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cherqui D, Soubrane O, Husson E, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet 2002;359:392-6. [Crossref] [PubMed]
- Scatton O, Katsanos G, Boillot O, et al. Pure laparoscopic left lateral sectionectomy in living donors: from innovation to development in France. Ann Surg 2015;261:506-12. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Ogiso S, Conrad C, Araki K, et al. Laparoscopic transabdominal with transdiaphragmatic access improves resection of difficult posterosuperior liver lesions. Ann Surg 2015;262:358-65. [Crossref] [PubMed]
- Tomishige H, Morise Z, Kawabe N, et al. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J Gastrointest Surg 2013;5:173-7. [Crossref] [PubMed]
- Liu CL, Fan ST, Cheung ST, et al. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg 2006;244:194-203. [Crossref] [PubMed]
- Kim KH, Kang SH, Jung DH, et al. Initial outcomes of pure laparoscopic living donor right hepatectomy in an experienced adult living donor liver transplant center. Transplantation 2017;101:1106-10. [Crossref] [PubMed]
- Suh KS, Hong SK, Lee KW, et al. Pure laparoscopic living donor hepatectomy: focus on 55 donors undergoing right hepatectomy. Am J Transplant 2018;18:434-43. [Crossref] [PubMed]
- Suh KS, Hong SK, Yi NJ, et al. Pure 3-dimensional laparoscopic extended right hepatectomy in a living donor. Liver Transpl 2016;22:1431-6. [Crossref] [PubMed]
- Rotellar F, Pardo F, Benito A, et al. Totally laparoscopic right hepatectomy for living donor liver transplantation: analysis of a preliminary experience on 5 consecutive cases. Transplantation 2017;101:548-54. [Crossref] [PubMed]
- Han HS, Cho JY, Yoon YS, et al. Total laparoscopic living donor right hepatectomy. Surg Endosc 2015;29:184. [Crossref] [PubMed]
- Nugroho A, Kim OK, Lee KW, et al. Evaluation of donor workups and exclusions in a single center experience of living donor liver transplantation. Liver Transpl 2017;23:614-24. [Crossref] [PubMed]
- Suh KS, Suh SW, Lee JM, et al. Recent advancements in and views on the donor operation in living donor liver transplantation: a single-center study of 886 patients over 13 years. Liver Transpl 2015;21:329-38. [Crossref] [PubMed]
- Hong SK, Lee KW, Kim HS, et al. Optimal bile duct division using real-time indocyanine green near-infrared fluorescence cholangiography during laparoscopic donor hepatectomy. Liver Transpl 2017;23:847-52. [Crossref] [PubMed]
- Hong SK, Lee KW, Choi Y, et al. Initial experience with purely laparoscopic living-donor right hepatectomy. Br J Surg 2018;105:751-9. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Hong SK, Suh KS, Kim HS, et al. Pure 3D laparoscopic living donor right hemihepatectomy in a donor with separate right posterior and right anterior hepatic ducts and portal veins. Surg Endosc 2017;31:4834-5. [Crossref] [PubMed]
- Ishizawa T, Bandai Y, Ijichi M, et al. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 2010;97:1369-77. [Crossref] [PubMed]
- Shehta A, Lee JM, Lee KW, et al. Pure laparoscopic living donor hepatectomy for donors with right portal vein anatomical variations. Liver Transpl 2019;25:1445-54. [Crossref] [PubMed]
Cite this article as: Lee JM, Lee KW, Hong K, Han ES, Hong SK, Yi NJ, Suh KS. Various techniques for bile duct division in pure laparoscopic donor hepatectomy. Laparosc Surg 2020;4:24.


