
The role of minimally invasive surgery in the treatment of polycystic liver disease
Introduction
Polycystic liver disease (PLD) is a rare disorder with an incidence lower than 0.01% and characterized by diffuse multiple cysts, arbitrarily 20 or more (1,2). PLD is the phenotypic expression of two possible inherited conditions: autosomal dominant polycystic kidney disease and autosomal dominant PLD, respectively accounting for 90% and 10% of PLD (3). The association between kidney and hepatic cysts was first described in 1856 (4). Recent publications report that 50% to 70% of patients with autosomal dominant polycystic kidney disease develop PLD, and independent risk factors for liver involvement include advanced patient age, female gender and sever kidney disease (5-7). Differently, the isolated form of PLD as a distinct autosomal dominant PLD was first suggested and confirmed much later, respectively in 1982 and 1996 (8,9).
Despite the general imprint is an asymptomatic enlarged liver, up to 20% of PLD patients become symptomatic: either few liver cysts tend to grow reaching considerable dimensions (>5 cm), or the liver volume tends to enlarge because of small but multiple cysts, with its texture becoming stiff (10-12). The most frequent symptoms are related to the hepatomegaly itself and/or the compression by large cyst on the stomach, duodenum and diaphragm, and include: abdominal or back pain, early fullness and bloating, nausea and loss of appetite, supine dyspnea and tiredness. In advanced cases, symptoms can also arise from compression of vasculo-biliary structures generating lower limb edema, ascites and obstructive cholestasis. Moreover, in 10–30% of patients, hemorrhage or infection of some dominant cysts can cause acute pain and pyrexia (13,14). Differently from renal involvement—where the decompressive treatment has anyway a beneficial effect on kidney function regardless of the presence of symptoms—invasive treatments of PLD are only indicated for symptomatic patients. In fact, pain and abdominal discomfort often recur and progressively become chronic, thus causing a significant deterioration of the quality of life (11,15). Surgical treatment options include fenestration (also termed deroofing or unroofing), liver resection and orthotopic liver transplantation (16). The superiority of operative non-surgical procedures (such as percutaneous aspiration with or without sclerosant injection, or arterial embolization) over surgical treatments is still to be clarified.
Minimally-invasive approaches for liver surgery have gained popularity over the last 20 years, and include pure laparoscopy, hand-assisted laparoscopic surgery, single-incision laparoscopic surgery and robotics (17-23). Almost all types of resections have been described, and technical adjustments and operative learning curves have been elaborated to provide guidance in accomplishing safe operations of increasing difficulty (24-42). Advantages for patients comprise decreased blood loss and transfusions, lower complication rates and shorter length of stay, along with adequate oncologic radicality (43-52).
In this review we describe the state of the art on and technical considerations of minimally-invasive approaches for the various surgical options for PLD.
Surgical treatments for PLD
In 1997 Gigot et al. classified PLD according to the number and size of the cysts and the amount of normal parenchyma (53). This classification is the most used and has the value to guide the choice between the possible surgical options. Other classifications were produced in the 2000’s, taking into consideration the presence of collateral venous circulation and symptoms (Schnelldorfer), or the number of cysts and hepatomegaly (Quian) (54,55). In 2018, the experience of the Boujon Hospital in Paris allowed to identify relevant criteria for a successful allocation of PLD patients to liver resection rather than transplantation (56). The morphological features of the PLD, the clinical presentation and the status of the liver function should be carefully evaluated so as to choose the best treatment for each patient among fenestration, liver resection and liver transplantation.
Cyst fenestration
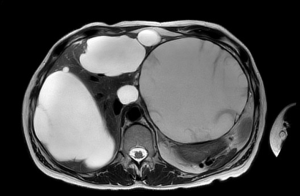
Cyst fenestration was first reported in the late 1960’s by Lin et al. and represented the unique curative treatment for PLD for many years (57). This procedure consists in the excision of the cyst wall till its boundaries with the liver tissue. It permits the drainage of the cyst fluid in the abdominal cavity (thus providing de-tension) and prevents the spontaneous reconstitution of its roof (thus reducing the possibility of recurrence). The ideal PLD setting to apply fenestration are patients with superficial cysts, large in dimension and limited in number (Gigot type 1) (Figure 1) (53). In fact, unroofing can relief the symptoms related to the steric hindrance from few enlarged cysts, rather than those related to the hepatomegaly from extensive occupation of the parenchyma from multiple small cyst. This procedure has the advantage of being relatively low-invasive, rapid and safe, and at the same time respectful of the surrounding liver parenchyma as well as of its major vasculo-biliary structures, which remain untouched.
Before the development of minimally-invasiveness, the open approach was the standard. However, with the technical progress, laparoscopic cyst fenestrations were up taken soon: those were the first procedures in liver surgery to be performed by laparoscopy, and then were increasingly described for patients with simple cysts and extended to PLD during the 1990’s (53,58-61). The basic surgical technique recalls that of open unroofing: it consists in 3–4 ports laparoscopy, aspiration of cyst fluid first and wide fenestration of the cyst wall up to the transition between the cyst roof and the liver parenchyma (Figure 2). Usually, the fenestration is carried out by the means of an energy device which can cut and seal simultaneously the thin layer of tissue covering the cyst cavity. The main operative advantage of laparoscopy is that of magnification which can provide a better field of vision for surgeons. Moreover, laparoscopy may decrease the operative challenges of future re-operations, that are not rare for PLD patients, given its recognized effect on minimizing intrabdominal adhesions (58,62).

However, the greater benefits of laparoscopic fenestrations relate to the postoperative short-term outcomes of patients. Some of studies on laparoscopic cyst deroofing for PLD have documented operative time and mortality rates similar to open, together with lower postoperative morbidity (53,59,61). In 1994 Morino et al. reported a median operative time of 202 minutes, two out of 7 patients complicated by postoperative ascites managed conservatively, and nihil mortality (60). In the series of Katkhouda et al. published in 1999, median operative time was 141 minutes, complication rate was 33%, and the median hospital stay was 3 days (63). In 2009 Gall et al. published the outcomes of 13 PLD patients treated with laparoscopic fenestration with a median operative time of 105 minutes and a 31% postoperative morbidity, which was higher than that for simple cysts (15%), but lower than open deroofing for PLD (40%) (64). In 2019 Bernts et al. performed a systematic review and meta-analysis of cohort studies and clinical trials focused on laparoscopic fenestrations of symptomatic cysts (65). On a total of 1,314 patients, 33% received laparoscopic cyst deroofing in the setting of PLD. Regarding the whole series of fenestrations, the median operative time was 83.5 minutes and conversion to open was 4.5% (mainly related to bleeding, unfavorable locations or intra-abdominal adhesions); postoperative morbidity was 10.8% (typically bile leakage, ascites, pleural effusion or infections) and median hospital stay was 5 days; the pooled estimates of major morbidity and procedure-related mortality after surgery were 3.3% and 1.0%, respectively. The authors undertook also a specific analysis for PLD patients, including 15 studies (for a total of 146 patients). For this subgroup, the pooled estimates of procedure-related mortality and major morbidity were similar to the whole series (2.3% and 7.2%). However, the conversion rate was higher if compared to the overall results (8.2%, even if not statistically significant) and postoperative complications were more frequent (29.3%). The authors explain that the potential causes of the elevated risk of complications are the changes in hepatic anatomy in PLD and the extensive use of fenestrations, with some studies describing the fenestration of over 30 cysts per patient (58,66).
The primary endpoint of Bernts meta-analysis was reporting also on symptomatic relief after laparoscopic fenestration of symptomatic cysts and the benefit on clinical symptoms (65). Despite the data were insufficient to meta-analyze the symptomatic relief specifically for PLD, the authors reported a pooled relief of symptoms of 90% on the whole series. However, the long-term effectiveness of this procedure is globally rare, and for many patients the need for reoperation occurs within 5 years. For example, in 2009 Gall et al. reported 85% of symptomatic recurrence and reoperations after laparoscopic fenestrations higher for PLD (85% and 46%) than simple cysts (29% and 4%) on a median follow up of 77 months (64). Moreover, the symptomatic recurrence after laparoscopic fenestrations for PLD were more frequent than open (55%), even if not statistically significant. In general, only few studies and limited in the number of patients have analyzed the long-term efficacy of laparoscopic cyst fenestration in the setting of multiple cysts (59,61,67,68). Thus, in 2018 Zhang and colleagues conducted a systematic review and meta-analysis of studies aimed at exploring the long-term efficacy of laparoscopic cyst deroofing based on the recurrence rates (69). Thirty-one studies were globally considered, and the four studies reporting on patients affected by multiple cysts were meta-analyzed separately (59,61,67,68). The results showed a non-significant difference in recurrence rates between laparoscopic and open fenestrations (19.5% and 16.6% respectively). The authors concluded that laparoscopic fenestration has favorable short- and long-term effects, justifying its extensive application in clinical settings of regardless of solitary or multiple hepatic cysts. The long-term efficacy after laparoscopic fenestration was meta-analyzed also by Bernts et al., this time considering the clinical recurrence of symptoms and need for reintervention (65). In the whole series, 62 studies with a mean follow-up of 30 months were included in the analysis. The authors found that long-term symptomatic relief is less well achieved in PLD: compared to the overall series, the pooled analysis of both the clinical recurrence of symptoms and the rate of reintervention resulted significantly higher for PLD (33.7% versus 9.6%, and 26.4% versus 7.1%).
The temporarily efficacy of multiple fenestration is frequently linked to incomplete treatment due to unfavorable cyst locations. Despite central cysts can also be fenestrated through opened superficial cysts, a deep location usually limits the completeness of the unroofing. Similarly, cysts located at the dome of the liver tend to develop adhesions to the diaphragm, and to recur rapidly through the constitution of a neo-cyst. Thus, a relevant issue is whether the topography of the cyst is determinant for the efficacy on short- and long-term symptomatic recurrence, and should be considered important in the choice of the technique. Indeed, patients with the target cyst in Segments 6, 7 and 8 or located centrally in the parenchyma are recognized as the most difficult to be visualized and reached by laparoscopic instruments. Therefore, similarly to resection of solid liver lesions, a wide-enough laparoscopic fenestration can be more challenging for posterosuperior than anterolateral segments when performed by laparoscopy. Not surprisingly, it has been reported that laparoscopic access to the posterior and superior part of the liver is almost impossible in PLD patients and that unfavorably located cysts have a higher tendency for recurrence (53,58,60,67). In view of this evidence, most of the authors highlight the importance of adequate patient’s selection for a laparoscopic approach to ensure satisfactory outcomes in terms of recurrence rates: laparoscopic deroofing should be used cautiously for patients with deep or posterosuperior dominant cysts, while should be considered a valid option for fenestration of large, symptomatic liver cysts located in the anterolateral segments.
The limited long-term efficacy is also due to a predisposition to developing new cysts. In fact, hepatic cysts, regardless whether they are solitary or multiple, result from the inactivation of 2 alleles from PLD genes. Being PLD a genetic disorder, patients already have a germline mutation and must acquire only one additional somatic mutation to develop the phenotype (70). As a consequence, the natural progression of PLD frequently overtakes the potential volume-curtailing effect of previous fenestrations, and renders the treatment often temporarily. Some authors also highlight the need that future studies set the follow up of recurrence using standard imaging at fixed time points and specific questionnaires such as the PLD-Q or POLCA (70). This would allow the identification of false positives (patients with recurrence of symptoms without evidence of radiological recurrence) and to achieve better evidence on long-term efficacy. However, considering that symptoms are mainly related to large cysts and in view of the limited long-term efficacy and higher risks, a sensible approach is to keep high the threshold for re-do laparoscopic fenestrations in PLD.
For all those cases where laparoscopic fenestration does not apply well, more invasive but more definitive surgical operations are possible options.
Liver resection
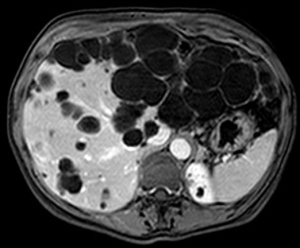
Hepatic resection for PLD was first reported by in the mid 1990’s by Que and colleagues (71). This treatment is indicated for patients with symptomatic enlargement of a certain area of the liver caused by massive occupation from multiple small cysts, and residual wide areas of normal parenchyma (Gigot type 2) (Figure 3) (53).
As for what concerns laparoscopic liver resections for PLD, only scattered cases as part of mixed series have been reported. As an example, in 2003 Descottes and colleagues published a multicenter experience on laparoscopic liver resections for benign conditions, which included two liver resections for PLD (72). In 2004 Andoh et al. described a pure laparoscopic right hemihepatectomy for right predominant PLD: operation time was 320 minutes, intraoperative blood loss was 120 mL, and postoperative course was uneventful (73). More recently, few laparoscopic left lateral sectionectomies were reported in the publications of Choi et al. (2013) and Martinez-Perez (2016), the latter describing also one case of right posterior sectionectomy (74,75). No specific series of laparoscopic liver resections or comparative studies are available yet for PLD.
Laparoscopic liver resections for PLD may be challenging due to the distortion of the liver anatomy from the cyst and the consequent difficulty in following the correct transection planes preserving the vital anatomical pedicles of the liver remnant (Figure 4). At the same time, little parenchymal resection is often needed because of multiple cysts, with consequent lower risk of blood loss. In this regard, the laparoscopic technique can have pros and cons with respect to other indications, and certainly some peculiar technical features.
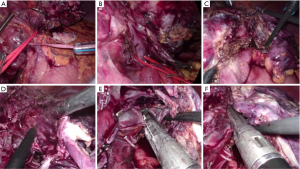
Despite the paucity of reports in this specific setting, it is worth noting that no publications have suggested that PLD is a suboptimal indication or a contraindication for a laparoscopic liver resection. On the other hand, as for any other benign disease, is warranted an attitude that does not expand the indication to liver resection in view of the adoption of a minimally-invasive approach.
Other surgical treatments
Liver transplantation can be a valid option for PLD patients with hepatomegaly due to multiple and disseminated small bilobar cysts (Gigot type 3) (53). While donor hepatectomies are the most recent advance of minimally-invasive liver surgery and their feasibility and safety has been recently acknowledged, orthotopic liver transplantation remains a prerogative of open surgery for all indications.
Minimally-invasive innovations include surgical procedures performed with a robotic approach, which may help in overcoming some limits of conventional laparoscopy. The major operative advantages regard: an easier depth perception of the visual field, a better access to narrow working spaces on postero-superior segments, and a facilitation in suturing vessels or biliary ducts. The stability of robotics platforms, the availability of highly-maneuverable wristed instruments and 3-dimensional visualization systems favor the completion of dissection, biliostasis and hemostasis in all hepatic segments. For PLD, these features are especially useful for dissecting meticulously those cysts located in segment 7 or 8 with adhesion to the diaphragm, or close to the hilar pedicles, as highlighted by Tsirlis et al. (76). In 2018 they have published a series of 17 robotic fenestrations, which is the largest so far. Even if only one procedure was in the setting of PLD, the authors concluded they would expect lower recurrence rates in the future for postero-superior deroofing, and this may apply well to PLD patients given their trend toward reiterative treatments. However, both robotic fenestrations and liver resections remain anecdotal and described as part of mixed series or isolated case reports. Goja et al. have reported two robotic surgeries for PLD: a left lateral segment partial cystectomy associated with segment 4 cyst deroofing, with a postoperative stay of 4 days; a left hepatectomy with concomitant right-sided cyst deroofing, with a postoperative course complicated by seizures and thrombotic thrombocytopenic purpura and thus 28 days long (77). Additionally, Nota et al. have described a robotic fenestration in a PLD patient with predominant cysts in the right subphrenic area and epigastric region (78). The procedure was carried out with acceptable operating times, minimal blood loss and regular recovery. Given the shortage of reports, no firm conclusions can be drawn regarding the short-term outcomes of the robotic approach for PLD surgery, nor for the potential beneficial effect on recurrence. Considering the increased costs of robotics, further studies are necessary before promoting its adoption on a larger scale.
In 2016 Choi and colleagues have reported two successful single-port laparoscopic deroofing procedures (74). In the same year, Sumer et al. described the fenestration in a PLD patient through the combination of mini-laparoscopic incisions and trans-gastric endoscopic removal of the specimens (79). The patient was discharged in postoperative day 4 without complications. Mini-laparoscopic surgery has the objective of minimizing the abdominal wall incisions reducing the surgical trauma. The association with endoscopy can allow safe intrabdominal excision and easy extraction if the resected and extraction organ are located in the same area (as the liver and stomach are). As for other abdominal operations, the use of a mini-laparoscopic approach and endoscopy for PLD deroofing has resulted in an even less invasive procedure.
Interestingly, Tanaka et al. have described the application of the laparoscopic indocyanine green fluorescence imaging, highlighting its specific advantages for PLD fenestrations (80). This advanced modality of intraoperative imaging can give confirmation that the cyst does not contain bile, may allow a precise adjustment of the resection line after visualization of bile branches, and a fine detection of small bile leaks at the end of the procedure facilitating their suture.
Despite the feasibility of these alternative approaches, their added value over conventional laparoscopy are uncertain and worth to be investigated further before their diffusion.
Evolution of minimally-invasive surgery for PLD
An important issue is whether the evolution of laparoscopic surgery is complete yet, or still ongoing. For fenestrations only, Bernts et al. did not find a significant change in conversion rates, incidence of morbidity and length of stay over time if performed by laparoscopy (studies published before or after 2005) (65). Similarly, symptomatic relief and recurrence rates were similar among the two periods of the meta-analysis. The authors only highlight that conversion rates above 10% occurred exclusively before 2006. Some additional data on both fenestrations and liver resections are provided by Antonacci et al. through a systematic review published in 2014 (81). The authors carried out a qualitative analysis dividing 30 selected studies on laparoscopic and open procedures into three periods in relation to the development of laparoscopic surgery: P1 “pioneering period of laparoscopy”; P2 “period of the development of laparoscopy”; P3 “period of the diffusion of laparoscopy”. On a total of 948 patients, 206 had PLD. In P1 the preferred approach was open (66.3%), the global conversion rate of laparoscopic fenestrations was 18.1% and all the patients with PLD were converted to open. In P2 the effect of technological progress was evident: laparoscopy reached 56.7% of approaches, and both the global and PLD conversion rates decreased significantly to 2.3% and 66.6% respectively. In P3 the completion of learning curves was beneficial: laparoscopic was confirmed as the preferred approach (69.9%) and the global conversion rate remained low (5.6%) (due to the paucity of PLD cases, no information was provided regarding conversion rates in this subgroup of patients). Globally, in all three periods laparoscopy showed a significant reduction in operative time and hospital stay. Also, the complication rate in the laparoscopic group showed a trend toward a progressive reduction, despite a significant difference was not seen (27.2% in P1, 1.3% in P2, 9.7% in P3). Moreover, the rate of recurrence for laparoscopy was significantly lower in P3 (6.1%) if compared to both P1 and P2 (36.3% and 17.4%). However, 70–80% of symptomatic recurrences developed constantly in PLD patients. The authors concluded that, the laparoscopic approach could be the treatment of choice in patients with non-parasitic liver cysts, as this approach would ensure the typical advantages of minimally invasive surgery, such as reduced hospital stay and less postoperative pain. Recurrence seemed to be associated with the presence of PLD rather than with the type of technique used, since laparoscopic treatment provided acceptable recurrence rates and comparable to those of conventional surgery.
Evolution of evidence on laparoscopic liver surgery for PLD
PLD is an infrequent surgical indication and the literature on minimally-invasive approaches on this topic lacks of high-quality studies. The majority of the evidence on laparoscopic deroofing is supported by single-center retrospective series and few comparative studies. Scattered laparoscopic liver resections have been reported and no randomized controlled trials (RCTs) have compared minimally-invasive versus open approaches in this setting. Also, data on recurrence are supported by a non-standardized reporting of outcomes, diversity in imaging modalities and timing for follow up, variability in the detection of symptoms, which remain all a significant cause of heterogeneity. Moreover, specific recommendations in favor or against PLD minimally-invasive treatments were not produced by expert consensus conferences or guidelines meeting held during the last 10 years (82-85). Since the literature on laparoscopic liver surgery has a general trend toward improving the evidence levels (increasing prospective series, matched comparative studies and some RCTs, efforts should be put collectively to produce studies of higher quality in the specific context of PLD, potentially with multicentric data collections (86-88).
Conclusions
Laparoscopic cyst fenestrations for PLD appear as a valid alternative to open surgery for patients with Gigot type 1 PLD and dominant cysts located in the anterolateral segments. This approach may confer several operative advantages for surgeons, and have a beneficial effect on the postoperative course of patients. However, candidates should be carefully selected, since the adequacy for deeply located cysts or in the postero-superior segments is associated with an increased rate of recurrence than open. The laparoscopic approach appears promising for liver resection in the setting of Gigot type 2 PLD, but peculiar technical issues should be considered as potential challenges. Specific reports on their effectiveness are scant, and should be studied further. Other approaches such as robotics, single-incision laparoscopy and hand-assisted may be alternatives to pure laparoscopy; however, their added value and increased costs should be considered before their application as a standard minimally-invasive technique.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Francesco Ardito) for the series “The Role of Minimally Invasive Liver Surgery to Treat Hepatic Benign Disease” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2020.03.06). The series “The Role of Minimally Invasive Liver Surgery to Treat Hepatic Benign Disease” was commissioned by the editorial office without any funding or sponsorship. FR serves as an unpaid editorial board member of Laparoscopic Surgery from Aug 2020 to Jul 2022. LA serves as an unpaid editorial board member of Laparoscopic Surgery from December 2019 to November 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nat Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol 2013;10:101-8. [Crossref] [PubMed]
- Drenth JP, Chrispijn M, Nagorney DM, et al. Medical and surgical treatment options for polycystic liver disease. Hepatology 2010;52:2223-30. [Crossref] [PubMed]
- Temmerman F, Missiaen L, Bammens B, et al. Systematic review: the pathophysiology and management of polycystic liver disease. Aliment Pharmacol Ther 2011;34:702-13. [Crossref] [PubMed]
- Bristowe F. Cystic disease of the liver associated with similar disease of the kidneys. Trans Pathol Soc Lond 1856;7:229-34.
- D'Agata ID, Jonas MM, Perez-Atayde AR, et al. Combined cystic disease of the liver and kidney. Semin Liver Dis 1994;14:215-28. [Crossref] [PubMed]
- Gabow PA, Johnson AM, Kaehny WD, et al. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 1990;11:1033-7. [Crossref] [PubMed]
- Sherstha R, McKinley C, Russ P, et al. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology 1997;26:1282-6. [PubMed]
- Berrebi G, Erickson RP, Marks BW. Autosomal dominant polycystic liver disease: a second family. Clin Genet 1982;21:342-7. [Crossref] [PubMed]
- Karhunen PJ, Tenhu M. Adult polycystic liver and kidney diseases are separate entities. Clin Genet 1986;30:29-37. [Crossref] [PubMed]
- Vauthey JN, Maddern GJ, Blumgart LH. Adult polycystic disease of the liver. Br J Surg 1991;78:524-7. [Crossref] [PubMed]
- Grünfeld JP, Albouze G, Jungers P, et al. Liver changes and complications in adult polycystic kidney disease. Adv Nephrol Necker Hosp 1985;14:1-20. [PubMed]
- Bistritz L, Tamboli C, Bigam D, et al. Polycystic liver disease: experience at a teaching hospital. Am J Gastroenterol 2005;100:2212-7. [Crossref] [PubMed]
- Misra A, Loyalka P, Alva F. Portal hypertension due to extensive hepatic cysts in autosomal dominant polycystic kidney disease. South Med J 1999;92:626-7. [Crossref] [PubMed]
- Telenti A, Torres VE, Gross JB, et al. Hepatic cyst infection in autosomal dominant polycystic kidney disease. Mayo Clin Proc 1990;65:933-42. [Crossref] [PubMed]
- Redston MS, Wanless IR. The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies corrected. Mod Pathol 1996;9:233-7. [PubMed]
- Grimm PC, Crocker JF, Malatjalian DA, et al. The microanatomy of the intrahepatic bile duct in polycystic disease: comparison of the cpk mouse and human. J Exp Pathol (Oxford) 1990;71:119-31. [PubMed]
- Cipriani F, Ratti F, Aldrighetti L. Laparoscopic liver resections at the gates of 2020: a stand-alone field of hepatobiliary surgery. Hepatobiliary Surg Nutr 2020;9:371-3. [Crossref] [PubMed]
- Aldrighetti L, Belli G, Boni L, et al. Italian experience in minimally invasive liver surgery: a national survey. Updates Surg 2015;67:129-40. [Crossref] [PubMed]
- Berardi G, Van Cleven S, Fretland ÅA, et al. Evolution of Laparoscopic Liver Surgery from Innovation to Implementation to Mastery: Perioperative and Oncologic Outcomes of 2,238 Patients from 4 European Specialized Centers. J Am Coll Surg 2017;225:639-49. [Crossref] [PubMed]
- Fiorentini G, Swaid F, Cipriani F, et al. Propensity Score-Matched Analysis of Pure Laparoscopic Versus Hand-Assisted/Hybrid Major Hepatectomy at Two Western Centers. World J Surg 2019;43:2025-37. [Crossref] [PubMed]
- Cipriani F, Catena M, Ratti F, et al. LESS technique for liver resection: the progress of the mini-invasive approach: A single-centre experience. Minim Invasive Ther Allied Technol 2012;21:55-8. [Crossref] [PubMed]
- Aldrighetti L, Ratti F, Catena M, et al. LaparoEndoscopic Single Site (LESS) surgery for left lateral hepatic sectionectomy as an alternative to traditional laparoscopy: a case-matched analysis from a single center. Surg Endosc 2012;26:2016-22. [Crossref] [PubMed]
- Patriti A, Cipriani F, Ratti F, et al. Robot-assisted versus open liver resection in the right posterior section. JSLS 2014;18:e2014.00040.
- Aldrighetti L, Pulitanò C, Catena M, et al. A prospective evaluation of laparoscopic versus open left lateral hepatic sectionectomy. J Gastrointest Surg 2008;12:457-62. [Crossref] [PubMed]
- Smerieri N, Fiorentini G, Ratti F, et al. Laparoscopic left hepatectomy for mucinous cystic neoplasm of the liver. Surg Endosc 2018;32:1068-9. [Crossref] [PubMed]
- Cipriani F, Ratti F, Cardella A, et al. Laparoscopic Versus Open Major Hepatectomy: Analysis of Clinical Outcomes and Cost Effectiveness in a High-Volume Center. J Gastrointest Surg 2019;23:2163-73. [Crossref] [PubMed]
- Cipriani F, Ratti F, Fiorentini G, et al. Effect of previous abdominal surgery on laparoscopic liver resection. Analysis of feasibility and risk factors for conversion. J Laparoendosc Adv Surg Tech A 2018;28:785-91. [Crossref] [PubMed]
- Cipriani F, Ratti F, Paganelli M, et al. Laparoscopic or open approaches for posterosuperior and anterolateral liver resections? A Propensity Score based analysis of the degree of advantage. HPB (Oxford) 2019;21:1676-86. [Crossref] [PubMed]
- Piccolo G, Ratti F, Cipriani F, et al. Totally laparoscopic radical cholecystectomy for gallbladder cancer: a single center experience. J Laparoendosc Adv Surg Tech A 2019;29:741-6. [Crossref] [PubMed]
- Cipriani F, Alzoubi M, Fuks D, et al. Pure laparoscopic versus open hemihepatectomy: a critical assessment and realistic expectations. A propensity score-based analysis of right and left hemihepatectomies from 9 European tertiary referral centers. J Hepatobiliary Pancreat Sci 2020;27:3-15. [Crossref] [PubMed]
- Fiorentini G, Ratti F, Cipriani F, et al. Theory of Relativity for Posterosuperior Segments of the Liver. Ann Surg Oncol 2019;26:1149-57. [Crossref] [PubMed]
- van der Poel MJ, Barkhatov L, Fuks D, et al. Multicentre propensity score matched study on laparoscopic versus open repeat liver resection for colorectal liver metastases. Br J Surg 2019;106:783-9. [Crossref] [PubMed]
- Fiorentini G, Ratti F, Cipriani F, et al. Tips and Tricks for a Laparoscopic Approach to Paracaval Liver Segments. Ann Surg Oncol 2018;25:1695-8. [Crossref] [PubMed]
- Ratti F, Cipriani F, Catena M, et al. Approach to hepatocaval confluence during laparoscopic right hepatectomy: three variations on a theme. Surg Endosc 2017;31:949. [Crossref] [PubMed]
- Cipriani F, Shelat VG, Rawashdeh M, et al. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J Am Coll Surg 2015;221:265-72. [Crossref] [PubMed]
- Fiorentini G, Ratti F, Cipriani F, et al. Challenges and Technical Innovations for an Effective Laparoscopic Lymphadenectomy in Liver Malignancies. J Laparoendosc Adv Surg Tech A 2019;29:72-5. [Crossref] [PubMed]
- Aldrighetti L, Cipriani F, Fiorentini G, et al. A stepwise learning curve to define the standard for technical improvement in laparoscopic liver resections: complexity-based analysis in 1032 procedures. Updates Surg 2019;71:273-83. [Crossref] [PubMed]
- Halls MC, Alseidi A, Berardi G, et al. A Comparison of the Learning Curves of Laparoscopic Liver Surgeons in Differing Stages of the IDEAL Paradigm of Surgical Innovation: Standing on the Shoulders of Pioneers. Ann Surg 2019;269:221-8. [Crossref] [PubMed]
- Ratti F, Barkhatov LI, Tomassini F, et al. Learning curve of self-taught laparoscopic liver surgeons in left lateral sectionectomy: results from an international multi-institutional analysis on 245 cases. Surg Endosc 2016;30:3618-29. [Crossref] [PubMed]
- van der Poel MJ, Besselink M, Cipriani F, et al. Outcome and Learning Curve in 159 Consecutive Patients Undergoing Total Laparoscopic Hemihepatectomy. JAMA Surg 2016;151:923-8. [Crossref] [PubMed]
- Berardi G, Aghayan D, Fretland A, et al. Multicentre analysis of the learning curve for laparoscopic liver resection of the posterosuperior segments. Br J Surg 2019;106:1512-22. [Crossref] [PubMed]
- Halls MC, Berardi G, Cipriani F, et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg 2018;105:1182-91. [Crossref] [PubMed]
- Cipriani F, Rawashdeh M, Ahmed M, et al. Oncological outcomes of laparoscopic surgery of liver metastases: a single-centre experience. Updates Surg 2015;67:185-91. [Crossref] [PubMed]
- Cipriani F, Rawashdeh M, Stanton L, et al. Propensity score based analysis of outcomes of laparoscopic versus open liver resection for colorectal metastases. Br J Surg 2016;103:1504-12. [Crossref] [PubMed]
- Ratti F, Fiorentini G, Cipriani F, et al. Laparoscopic vs Open Surgery for Colorectal Liver Metastases. JAMA Surg 2018;153:1028-35. [Crossref] [PubMed]
- Ciria R, Ocaña S, Gomez-Luque I, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc 2020;34:349-60. [Crossref] [PubMed]
- Cipriani F, Fantini C, Ratti F, et al. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the indication in cirrhotic patients? Surg Endosc 2018;32:617-26. [Crossref] [PubMed]
- Ciria R, Gomez-Luque I, Ocana S, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for hepatocellular carcinoma. Updated results from the European Guidelines Meeting on Laparoscopic Liver Surgery, Southampton, UK, 2017. Ann Surg Oncol 2019;26:252-63. [Crossref] [PubMed]
- Aldrighetti L, Guzzetti E, Pulitanò C, et al. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 2010;102:82-6. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma: a propensity-score based case-matched analysis from a single institution. Surg Endosc 2016;30:1999-2010. [Crossref] [PubMed]
- Ratti F, Fiorentini G, Cipriani F, et al. Perioperative and Long-Term Outcomes of Laparoscopic Versus Open Lymphadenectomy for Biliary Tumors: A Propensity-Score-Based, Case-Matched Analysis. Ann Surg Oncol 2019;26:564-75. [Crossref] [PubMed]
- Ardito F, Aldrighetti L, Guglielmi A, et al. Surgical Management of Hepatic Benign Disease: Have the Number of Liver Resections Increased in the Era of Minimally Invasive Approach? Analysis from the I Go MILS (Italian Group of Minimally Invasive Liver Surgery) Registry. J Gastrointest Surg 2020;24:2233-43. [Crossref] [PubMed]
- Gigot JF, Jadoul P, Que F, et al. Adult polycystic liver disease: is fenestration the most adequate operation for long-term mana- gement? Ann Surg 1997;225:286-94. [Crossref] [PubMed]
- Schnelldorfer T, Torres VE, Zakaria S, et al. Polycystic liver disease: a critical appraisal of hepatic resection, cyst fenestration and liver transplantation. Ann Surg 2009;250:112-18. [Crossref] [PubMed]
- Qian Q, Li A, King BF, et al. Clinical profile of autosomal dominant polycystic liver disease. Hepatology 2003;37:164-71. [Crossref] [PubMed]
- Aussilhou B, Dokmak S, Dondero F, et al. Treatment of polycystic liver disease. Update on the management. J Visc Surg 2018;155:471-81. [Crossref] [PubMed]
- Lin TY, Chen CC, Wang SM. Treatment of non-parasitic cystic disease of the liver: a new approach to therapy with polycystic liver. Ann Surg 1968;168:921-7. [Crossref] [PubMed]
- Kabbej M, Sauvanet A, Chauveau D, et al. Laparoscopic fenestration in polycystic liver disease. Br J Surg 1996;83:1697-701. [Crossref] [PubMed]
- Koperna T, Vogl S, Satzinger U, et al. Nonparasitic cysts of the liver: results and options of surgical treatment. World J Surg 1997;21:850-4; discussion 854-5. [Crossref] [PubMed]
- Morino M, De Giuli M, Festa V, et al. Laparoscopic management of symptomatic nonparasitic cysts of the liver. Indications and results. Ann Surg 1994;219:157-64. [Crossref] [PubMed]
- Martin IJ, McKinley AJ, Currie EJ, et al. Tailoring the management of nonparasitic liver cysts. Ann Surg 1998;228:167-72. [Crossref] [PubMed]
- Aussilhou B, Douflé G, Hubert C, et al. Extended liver resection for polycystic liver disease can challenge liver transplantation. Ann Surg 2010;252:735-43. [Crossref] [PubMed]
- Katkhouda N, Hurwitz M, Gugenheim J, et al. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg 1999;229:460-6. [Crossref] [PubMed]
- Gall TMH, Oniscu GC, Madhavan K, et al. Surgical management and long-term follow-up of non-parasitic hepatic cysts. HPB (Oxford) 2009;11:235-41. [Crossref] [PubMed]
- Bernts LHP, Echternach SG, Kievit W, et al. Clinical response after laparoscopic fenestration of symptomatic hepatic cysts: a systematic review and meta-analysis. Surg Endosc 2019;33:691-704. [Crossref] [PubMed]
- Konstadoulakis MM, Gomatos IP, Albanopoulos K, et al. Laparoscopic fenestration for the treatment of patients with severe adult polycystic liver disease. Am J Surg 2005;189:71-5. [Crossref] [PubMed]
- Gigot JF, Metairie S, Etienne J, et al. The surgical management of congenital liver cysts. Surg Endosc 2001;15:357-63. [Crossref] [PubMed]
- Mazza OM, Fernandez DL, Pekolj J, et al. Management of nonparasitic hepatic cysts. J Am Coll Surg 2009;209:733-9. [Crossref] [PubMed]
- Zhang JY, Liu Y, Liu HY, et al. Comparison of the Recurrence Rates of Nonparasitic Hepatic Cysts Treated With Laparoscopy or With Open Fenestration: A Meta-Analysis Surg Laparosc Endosc Percutan Tech 2018;28:67-72. [PubMed]
- van Aerts RMM, van de Laarschot LFM, Banales JM, et al. Clinical management of polycystic liver disease. J Hepatol 2018;68:827-37. [Crossref] [PubMed]
- Que F, Nagorney DM, Gross JB, et al. Liver resection and cyst fenestration in the treatment of severe polycystic liver disease. Gastroenterology 1995;108:487-94. [Crossref] [PubMed]
- Descottes B, Glineur D, Lachachi F, et al. Laparoscopic liver resection of benign liver tumors. Surg Endosc 2003;17:23-30. Erratum in: Surg Endosc 2003;17:668. [Crossref] [PubMed]
- Andoh H, Sato T, Yasui O, et al. Laparoscopic right hemihepatectomy for a case of polycystic liver disease with right predominance. J Hepatobiliary Pancreat Surg 2004;11:116-8. [Crossref] [PubMed]
- Choi CJ, Kim YH, Roh YH, et al. Management of giant hepatic cysts in the laparoscopic era. J Korean Surg Soc 2013;85:116-22. [Crossref] [PubMed]
- Martinez-Perez A, Alberola-Soler A, Domingo-Del Pozo C, et al. Laparoscopic surgery and polycystic liver disease: Clinicopathological features and new trends in management. J Minim Access Surg 2016;12:265-70. [Crossref] [PubMed]
- Tsirlis T, Thakkar R, Sen G, et al. Robotic fenestration of massive liver cysts using EndoWrist technology. Int J Med Robot 2019;15:e1994 [Crossref] [PubMed]
- Goja S, Singh MK, Soin AS. Robotics in hepatobiliary surgery-initial experience, first reported case series from India. Int J Surg Case Rep 2017;33:16-20. [Crossref] [PubMed]
- Nota CL, Molenaar IQ, Borel Rinkes IH, et al. Robot-assisted Laparoscopic Fenestration of Giant Hepatic Cysts. Surg Laparosc Endosc Percutan Tech 2015;25:e163-5. [Crossref] [PubMed]
- Sumer F, Kayaalp C, Polat Y, et al. Transgastric removal of a polycystic liver disease using mini-laparoscopic excision. Interv Med Appl Sci 2016;8:89-92. [Crossref] [PubMed]
- Tanaka M, Inoue Y, Mise Y, et al. Laparoscopic deroofing for polycystic liver disease using laparoscopic fusion indocyanine green fluorescence imaging. Surg Endosc 2016;30:2620-3. [Crossref] [PubMed]
- Antonacci N, Ricci C, Taffurelli G, et al. Systematic review of laparoscopic versus open surgery in the treatment of non-parasitic liver cysts. Updates Surg 2014;66:231-8. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville Statement. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Liu R, Wakabayashi G, Kim HJ, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol 2019;25:1432-44. [Crossref] [PubMed]
- retland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 2018;267:199-207.
- Wong-Lun-Hing EM, van Dam RM, van Breukelen GJ, et al. Randomized clinical trial of open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery after surgery programme (ORANGE II study). Br J Surg 2017;104:525-35. [Crossref] [PubMed]
- U.S. National Library of Medicine. The ORANGE II PLUS - trial: open versus laparoscopic hemihepatectomy. Available online: https://clinicaltrials.gov/ct2/show/NCT01441856
Cite this article as: Cipriani F, Fiorentini G, Ratti F, Aldrighetti L. The role of minimally invasive surgery in the treatment of polycystic liver disease. Laparosc Surg 2020;4:42.


