Systematic review of laparoscopic fenestration and percutaneous sclerotherapy in the management of symptomatic nonparasitic simple liver cysts
Introduction
Among cystic hepatic diseases, nonparasitic simple liver cysts (N-SLC) represent a subgroup characterized by a smooth wall, covered with an epithelium secreting a fluid similar to bile. N-SLC are fluid-filled cavities that arise from malformations of the ductal plate during embryonic development (1). N-SLC are often incidentally diagnosed at imaging and their detection is gradually increasing, paralleling the increasing use of imaging modalities, including ultrasound, computed tomography, and magnetic resonance. In the majority of cases, N-SLC are asymptomatic but, as a result of their increase in volume, in about 15% of patients (2,3) they may be associated to aspecific symptoms, like abdominal pain, early satiety, nausea, or vomiting, becoming symptomatic (S)N-SLC (4). Depending on their diameter, which can be up to 30 cm, SN-SLC can also present as a palpable abdominal mass or as hepatomegaly.
When symptoms impair quality of life, treatment of SN-SLC is advocated and aims at relieving symptoms by reducing the cyst volume and consequently the compression on close structures. In this context, laparoscopic fenestration (LF) and percutaneous sclerotherapy (PS) represent the treatment options of choice. LF consists of a combination of cyst fluid aspiration, followed during the same operation by the excision of extra-hepatic cyst wall. PS consists in the radiologically guided placement of a pig tail drainage in the cyst cavity, followed by the aspiration of the cyst content, the injection in the cyst of a sclerosing agent in order to damage the inner epithelial layer of the cyst, and by the aspiration of the sclerosing agent by the cyst.
In 2014, Clinical American College of Gastroenterologists (ACG) guidelines suggested LF as the treatment option more effective, compared to PS, in the management of SN-SLC (5). In 2016 the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Guidelines on Interventional Ultrasound indicated, with a strong consensus, PS as a good alternative to LF, with lower complication rates and similar efficacy (6).
Given the lack of quantitative comparisons of LF versus PS results in the management of SN-SLC, we designed this systematic review of the available literature in order to assess efficacy, in terms of symptomatic relief and symptomatic recurrence, and safety of LF and PS.
Methods
Articles reporting characteristics of patients affected by SN-SLC and results following LF or PS were included in the current review. We included studies mainly focused on patients treated for solitary SN-SLC, however studies additionally including patients affected by multiple SN-SLC or symptomatic polycystic liver disease (SPLD) as a part of the study population were also included in the current review, while studies reporting uniquely on patients affected by SPLD were excluded from final analysis. Studies analyzing pediatric patients were excluded, as well as studies reporting on less than 5 patients. We systematically searched Medline (through PubMed) (7,8) for all years to August 2019 (last PubMed search was performed on August 25, 2019).
Initially, searches employing MeSH terms were performed for keywords and text (title or abstract). As shown in Table 1, search terms were organized in three main search groups, 4, 8, and 12, focused on patients affected by SN-SLC, patients undergoing LF treatment, and patients undergoing PS treatment, respectively. Combination of search group 4 singularly with search groups 8 and 12 led to the identification of 167 and 162 articles (search groups 9 and 13, respectively), focused on LF and on PS for SN-SLC, respectively. An additional “manual” research, using the “related articles” function, allowed to “explode” research. Further searches of reference lists of other articles supplemented the results of overmentioned research, resulting in the identification of additional 24 manuscripts (11 concerning LF and 13 concerning PS in the management of SN-SLC) and overall to the identification of 353 manuscripts, whose titles, abstracts, and full texts were independently reviewed by two authors (G Zimmitti and V Sega), in order to assess whether the studies met the eligibility criteria. Contrasting results between G Zimmitti and V Sega were discussed case by case, until an agreement was found. Included studies were classified, according to the management strategy, in two groups: LF and PS studies. An intention to treat analysis was performed, consequently cases converted to open procedures among LF studies were included in the analyses.
Table 1
| Search number | Search term | Results |
|---|---|---|
| 1 | Symptomatic liver cyst | 523 |
| 2 | Simple liver cyst | 596 |
| 3 | Nonparasitic liver cyst | 243 |
| 4 | 1 OR 2 OR 3 | 1,182 |
| 5 | Laparoscopic fenestration | 292 |
| 6 | Laparoscopic unroofing | 121 |
| 7 | Laparoscopic management | 23,679 |
| 8 | 5 OR 6 OR 7 | 23,924 |
| 9* | 4 AND 8 | 167 |
| 10 | Aspiration sclerotherapy | 370 |
| 11 | Percutaneous treatment | 27,716 |
| 12 | 10 OR 11 | 28,049 |
| 13# | 4 AND 12 | 162 |
*, studies concerning laparoscopic fenestration of nonparasitic symptomatic liver cysts; #, studies concerning percutaneous sclerotherapy for nonparasitic symptomatic liver cysts.
One author (G Zimmitti) extracted data from articles included in the study. In case of uncertainty concerning extracted data, two investigators (G Zimmitti and V Sega) discussed until resolution was achieved. Primary outcomes of the current study were relief of symptoms immediately after LF/PS, recurrence of symptoms requiring an additional treatment during follow-up, while secondary outcomes were study characteristics, patients characteristics, reintervention rates, operative time, hospital stay, conversion to laparotomy and surgical technique in case of LF, and sclerosant technique in case of PS. Morbidity following LF/PS was classified according to the Clavien-Dindo classification (9) by one investigator (G Zimmitti). Grade I and II were defined as minor complications and grade III, IV and V as major complications. The Newcastle-Ottawa scale (NOS) was used in order to assess the risk of bias within studies included in this review (10). According to NOS, a score was given to each included study and was based on selection of study group, inclusion of a control group, comparability of groups and outcome of interest clear definition. Studies were independently scored by G Zimmitti and V Sega and disagreements were resolved through discussion between two investigators.
Results
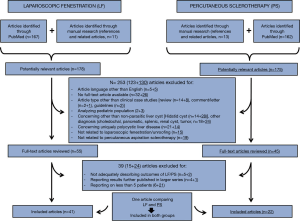
Of 353 manuscripts identified by Medline (through PubMed) and by manual research, 253 were initially excluded by title or abstract analysis, based on overmentioned eligibility and exclusion criteria, leading to 100 articles concerning LF (n=55) and PS (n=45) for the management of SN-SLC. Such manuscripts were reviewed by full text analysis, finally leading to the identification of 62 studies, 41 focused on LF [(11-35) and (36-51)] and 22 on PS (37,52-72). One article (37), comparing results of LF and PS, was included in both the two groups of papers. The content of such manuscripts was considered relevant for the current review (Figure 1).
The study design of manuscripts included in this review are shown in Table 2. Publication dates ranged between 1988 and 2019, study periods between 1985 and 2018. Of 1,678 patients included in the 62 overmentioned studies, 1,057 underwent a LF and 642 a PS for SN-SLC. Tables 3 and 4 respectively resumes characteristics of patients and results of studies reporting on LF and PS for patients affected by SN-SLC Among LF articles, patients affected by a solitary SN-SLC were 793 (75%), among PS articles were 425 (68%). Concerning study design of articles included in this review, in the LF and PS groups of articles retrospective studies were 40 (98%) and 14 (64%) while prospective studies were 1 (2%) and 5 (23%), respectively. In addition, in the PS group of articles 3 (14%) randomized clinical trial were observed. Mean NOS value was 5.4 for both LF and PS groups of articles. Median number of patients included in the reviewed articles was 21 both for LF (range, 5 to 66) and PS (range, 5 to 86) groups of articles. The main symptom related to SN-SLC was abdominal pain both in the two study groups. Follow-up duration following treatment was reported in 35 of 41 studies included in LF group and in 18 out of 22 studies included in the PS group. The main cyst diameter was reported in 33 studies (80%) in the LF group of articles and in 15 (68%) studies in the PS group of articles, however in 6 studies among PS articles it could be obtained by the main cyst volume, using the following formula: diameter = 3√(main cyst volume) × (π/6). Finally, main cyst volume average diameter was 12.2 cm among LF articles (7 to 18 cm) and 9.7 among PS articles. The location of treated SN-SLC was reported in 58% and 32% of LF and PS studies, respectively, and a right liver location was observed in 49% of cases among LF articles (25% to 100%) and 64% (26% to 76%). Ethanol was the only sclerosing agent in 14 (67%) out of 21 studies analyzing results of PS, in two studies ethanol or other sclerosing agents (acetic acid and tetracycline, respectively) were used, while in the remaining 5 studies sclerosing agent was represented by minocycline hydro-chloride (n=2), hypertonic saline solution (alone, n=1; associated with bleomycin, n=1), and polidocanol (n=1).
Table 2
| Study design | Laparoscopic fenestration (n=41) | Percutaneous sclerotherapy (n=22) |
|---|---|---|
| Retrospective study | 40 | 14 |
| Prospective study | 1 | 5 |
| Randomized clinical trial | 0 | 3 |
Table 3
| Author, year of publication | Study period | Study design | NOS | No. of patients (affected by SC, %) | Main symptom at diagnosis [%] | Main cyst size, cm | Right liver location | Surgery duration, minutes (conversion rate) | Morbidity (D-C, description of the most serious complication) | Symptom relief | LOS, days | Follow-up duration, months | Re-operation for symptoms recurrence (SC/MC) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Martin ( |
1988–1997 | RS | 6 | 20 [65] | Abd pain [NR] | 14 [8–24] | NR | 81 [45–180] (nil) | 25% (2, conservatively treated bile leak) | 100% | 3 [1–10] | 31 [1–80] | 30% (1/5) |
| Diez ( |
1992–1996 | RS | 5 | 10 [80] | Abd pain [NR] | NR [5–18] | 60% | NR (nil) | Nil | 100% | 4 [3–6] | NR [6–36] | 10% (0/1) |
| Zacherl ( |
1991–1998 | RS | 6 | 9 [100] | Abd pain/fullness [100] | NR | 100% | 87 [30–180] (nil) | 10% (2, upper extremity vein thrombosis) | 100% | 5 [3–13] | NR | 10% |
| Katkhouda ( |
1990–1997 | RS | 6 | 16 [100] | Abd pain [65] | 14 [7–22] | NR | 48 [45–56] (nil) | 6% (3A, abscess treated with CT guided drainage) | 100% | 1.3 [1–3] | 30 [3–78] | Nil |
| Hansman ( |
1984–2000 | RS | 4 | 6 [100] | Abd pain [NR] | 11.3±4.8 | 53% | NR (nil) | Nil | 100% | NR | 42±8 | Nil |
| Gigot ( |
1984–2001 | RS | 6 | 19 [53] | Abd pain [31] | 13 [8–30] | 63% | NR (21%) | 21% (3A, pneumothorax) | 100% | 6 [3–17] | 38.5 [NR] | 10% (0/2) |
| Regev ( |
1984–1999 | RS | 5 | 18 [100] | Abd pain/fullness [81] | NR | NR | NR | Nil | 100% | NR | 63 [6–90] | 5% |
| Schachter ( |
1996–1999 | RS | 5 | 12 [83] | Abd pain/fullness [100] | NR | 8% | NR | Nil | 100% | 5.8 [3–10] | 30 | Nil |
| Tocchi ( |
1975–1999 | RS | 6 | 8 [100] | Abd pain [NR] | 15±4.2 | NR | 78±20 (nil) | 12.5% (3B, reoperation for cyst wall bleeding) | NR | 6±5 | 50±9.3 | 25% |
| Tan ( |
1993–2001 | RS | 6 | 11 [91] | Abd pain/fullness [100] | 13 [6–18] | 90% | 68 [40–120] (nil) | Nil | 100% | 3 [2–4] | 20 [4–46] | 10% |
| Petri ( |
1982–2001 | RS | 4 | 34 [95] | Abd pain [81] | 10±3 | NR | NR | 9% [2] | NR | 7 | NR | 19.4% |
| Tagaya ( |
1993–1999 | RS | 6 | 5 [100] | Abd pain [100] | 9 [7–18] | 20% | 200 [72–270] (nil) | Nil | 100% | 9 [7–12] | 63 [35–102] | 20% |
| Fiamingo ( |
1996–2002 | RS | 6 | 15 [60] | Abd pain [81] | 10 [7–13] | NR | 80 [45–120] (7%) | 27% (3A, port site bleeding) | 100% | 5.5 [3–12] | 34 [2–72] | 12% (0/2) |
| Hsu ( |
1996–2001 | RS | 5 | 5 [20] | Abd fullness [100] | 18 [10–20] | NR | 150 [110–215] (nil) | Nil | 80% | 4 [3–5] | 48 [24–72] | Nil |
| Fabiani ( |
1989–2001 | RS | 5 | 40 [92] | Abd pain [NR] | 11 [5–20] | 47% | 82 [15–210] (5%) | Nil | 100% | 5.3 [2–12] | 69 [30–124] | 2% (0/1) |
| Neri ( |
1999–2003 | RS | 6 | 12 [58] | Abd pain [83] | 11 [6–20] | NR | 55 [40–90] (nil) | 17% (2, NR) | 100% | 6 [4–14] | 18 [3–38] | Nil |
| Palanivelu ( |
1995–2005 | RS | 6 | 27 [100] | Abd pain/fullness [100] | 16 [5–43] | 74% | 72 [55–104] (nil) | 11% (2, prolonged ascites conservatively treated) | 100% | 4 | 74 [2–144] | Nil |
| Szabó ( |
1995–2005 | RS | 5 | 21 [80] | NR | 7 [4.5–20] | NR | NR | Nil | 100% | 4.7 [3–37] | 49 [20–127] | 5% |
| Bai ( |
1998–2004 | RS | 5 | 44 [32] | NR | 12 [6–23] | 45% | NR | 11% (3A, bile leak treated with percutaneous drainage) | NR | 4±1.5 | 57 [24–103] | 4% (0/2) |
| Gall ( |
1985–2006 | RS | 5 | 48 [80] | Abd pain [87] | 11 [2–30] | 25% | 75 [40–170] (nil) | 15% (NR) | NR | 3 [NR] | 77 [3–250] | 4% |
| Gamblin ( |
2001–2008 | RS | 6 | 46 [13] | Abd pain [92] | 13 [2–21] | NR | 178 [54–380] (nil) | 19% (<3B, NR) | 100% | 2 [1–11] | 13 [1–49] | Nil |
| Mazza ( |
1990–2007 | RS | 6 | 46 [100] | Abd pain [57] | 9.2 [3–20] | 55% | NR | 4% (3A, bile leak treated with radiologic drainage) | 100% | 1.5 [1–3] | NR | 4.3% |
| Treckman ( |
1999–2007 | RS | 5 | 41 [79] | NR | 11 [6–18] | 59% | 85 [30–145] (7.3%) | 7% (3B, reoperation for bile leak) | NR | 5 [2–29] | 34 [6–103] | 3% |
| Loehe ( |
1995–2007 | RS | 6 | 66 [100] | Abd pain/fullness [81] | 10 | NR | 80±53 (1.5%) | 6% (3B, reoperation for cyst bleeding) | 90% | 5±3.7 | 69 [6–151] | 9% |
| Donati ( |
2002–2008 | RS | 4 | 21 [71] | Abd pain/tension [85] | 9.7±2.2 | 38% | 101 [55–165] (nil) | 5% (2, resp distress) | 100% | 4.2±2.6 | 40 [6–82] | 5% (0/1) |
| Faulds ( |
2009–2010 | PS | 5 | 6 [NR] | Abd pain [NR] | 11 [7–16] | 60% | NR (nil) | 17% (2, pneumonia) | 100% | 0 [0–5] | 10 [3–13] | Nil |
| Kim ( |
1997–2011 | RS | 4 | 14 [57] | NR | 10.5 | NR | 104 (7%) | Nil | NR | 3.3 | NR | NR |
| Wahba ( |
1999–2009 | RS | 5 | 23 [30] | NR | NR | NR | 12.2±3.2 (4%) | 4% (2, postoperative bleeding conservatively treated) | NR | NR | 59±40 | 4% |
| Mazoch ( |
1995–2009 | RS | 5 | 16 [NR] | Abd pain [87] | 15 [6–26] | 81% | NR | 12% (3B, reoperation for hemorrhage) | NR | 5.5 | 41 | 6% |
| Kamphues ( |
2002–2008 | RS | 5 | 43 [84] | NR | 8 [2–27] | NR | 94 [30–95] (nil) | Nil | 100% | 5 [2–8] | 49 [19–97] | 4.7% |
| Scheuerlein ( |
2000–2008 | RS | 6 | 47 [33] | Abd pain/fullness [73] | 12 [6–20] | NR | 80 [16–230] (6.4%) | 13% (NR) | 68% | 5.2 [2–19] | 43 [3–119] | 9% |
| Ardito ( |
2000–2010 | RS | 5 | 47 [60] | Abd pain [NR] | NR | 60% | 164 [50–240] (nil) | Nil | 100% | 5 [2–12] | 67 [12–142] | 2% (1/1) |
| Gocho ( |
2010–2011 | RS | 6 | 6 [100] | Abd fullness [NR] | 11 [10–15] | 50% | 144 [100–210] (nil) | Nil | 100% | 3 [3] | 15.5 [8–20] | Nil |
| Noerregaard ( |
2007–2012 | RS | 6 | 29 [100] | Abd pain [87] | NR | 55% | NR (6%) | 6% (3A, cyst hemorrhage requiring radiologic drainage) | NR | 1 [1–14] | 28 [1–60] | 16% |
| Lee ( |
2004–2014 | RS | 6 | 29 [64] | NR | 9.6±4.8 | NR | 110 [25–525] (nil) | 3% (2, pneumonia) | 100% | 5 [2–11] | 62 | 3% |
| Wu ( |
2009–2011 | RS | 5 | 30 [80] | NR | 11.5±3.2 | 30% | 58.5±6.8 (nil) | Nil | NR | 5±0.6 | 13±8 | Nil |
| Manterola ( |
2008–2015 | RS | 5 | 41 [NR] | NR | 10 [6–21] | 54% | 50 [35–90] (nil) | Nil | NR | 1 | 35 [6–90] | Nil |
| Debs ( |
2000–2012 | RS | 6 | 27 [60] | Abd pain [81] | 13.6 | 55% | NR | Nil | NR | NR | NR | 15% |
| Kisiel ( |
2002–2012 | RS | 4 | 48 [73] | NR | NR | NR | NR | 8% (3A, bile leak treated with ERCP) | 96% | 2 [1–7] | 66 [22–173] | 6% |
| de Reuver ( |
1999–2014 | RS | 8 | 34 [30] | Abd pain [66] | 12 [8–20] | 41% | 90 [60–120] (6%) | 20% (3B, reoperation for unspecified reason) | 85% | 5 [4–7] | 64 [15–165] | 9% |
| Tsirlis ( |
2016–2018 | RS | 6 | 17 [100] | Abd pain [76] | 15 [6–24] | 65% | 170 [97–335] (nil) | 12% (3A, bile leak treated with ERCP) | 100% | 2 [1–10] | 19 | Nil |
*, data concerning this manuscript refer to additional 5 patients who underwent laparoscopic liver resection (three left lateral sectionectomy, one right and one left hepatectomy); ç, data concerning this manuscript refer to additional 11 patients who underwent open cyst fenestration; &, included patients who underwent single-port LF. NOS, Newcastle Ottawa Scale; SC, solitary cyst; D-C, Dindo-Clavien; LOS, length of stay; MC, multiple cyst; RS, retrospective study; NR, not reported; abd, abdominal.
Table 4
| Author, year of publication | Study period | Study design | NOS | No. of patients (no. of cysts, % of patients with solitary cists) | Main symptom at diagnosis (%) | Right liver cyst (%) | Cyst size at diagnosis, cm (range) | Cyst volume at diagnosis, mL | Sclerosing agent | Morbidity (D-C) | Symptom relief | Cyst volume reduction at 12 months, % | LOS, days | Follow-up Duration, months | Re-treatment for symptoms recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kairaluoma ( |
1985–1987 | RS | 5 | 8 (15, 50%) | Abd pain [100] | NR | 10 [6–25] | NR | Ethanol | 12% (2, severe pain, procedure aborted) | 88% | NR | NR | 18 [12–32] | Nil |
| Montorsi ( |
1987–1991 | RS | 4 | 21 (21, 62%) | NR | 67 | NR | 450 | Ethanol | 10% [1] | 100% | 87.5 | NR | 18 [6–60] | NR |
| Tikkakoski ( |
1985–1992 | PS | 5 | 25 (59, 44%) | Abd pain [100] | NR | 9 [5–25] | 520 | Ethanol | 16% (1, pain during ethanol injection, procedure aborted) | 84% | 48 [12–72] | NR | 48 | NR |
| Cellier ( |
1992–1994 | RS | 5 | 7 (NR, 71%) | Abd pain [100] | NR | 8.5 [5–130] | NR | Minocycline, hydrochloride | 25% (1, transient pain) | 71% | NR | NR | 28 [24–42] | 43% |
| Poźniczek ( |
1995–2000 | RS | 4 | 19 (19, 100%) | NR | NR | 10 [5–24] | NR | Ethanol | NR | 58% | NR | NR | 35 [6–60] | 42% |
| Yang ( |
1997–2003 | RS | 7 | 27 (31, NR) | Abd pain [78] | NR | NR | 782 | Ethanol | 7% (2, hypotension requiring fluids/dopamine) | 100% | 98 | 1–3 | 29.6 | NR |
| Erdogan ( |
1997–2006 | RS | 5 | 30 (NR, 100%) | Abd pain [85] | NR | 13 [3.5–25] | NR | Ethanol/tetracycline | 7% (2, fever requiring readmission) | 100% | NR | 2 [2–23] | 15 [2–35] | 3% |
| Zerem ( |
1999–2004 | RCT | 7 | 20 (23, 75%) | Abd pain [100] | NR | NR | 175 | Ethanol | 6% (1, transient fever) | NR | 92 | 1 | 24 | 19% |
| Nakaoka ( |
1998–2007 | PS | 5 | 13 (17, 15%) | Abd pain [77] | NR | 8 [4.3–16] | 366 | Ethanol, amine oleate | 15% [1] | 100% | 93 [47–100] | NR | 54 [1–95] | 8% |
| Jusufovic ( |
NR | PS | 5 | 20 (20, 100%) | Abd pain [100] | 55 | NR | 718 | 20% saline solution | Nil | 100% | 96.3 [75–100] | NR | 24 | NR |
| Yan-Hong ( |
NR | RCT | 8 | 67 (NR, 100%) | Abd pain/discomfort [66] | – | 9 [8–13.5] | 429 | Ethanol | NR | 86% | 95 [NR] | NR | 30±4.4 | NR |
| Kim ( |
1997–2011 | RS | 4 | 14 (NR. 71%) | NR | NR | 11.5 | NR | Ethanol, acetic acid | 29% (5, death due to acetic acid intoxication) | NR | NR | 6 | NR | NR |
| Spârchez ( |
2008–2013 | PS | 5 | 13 (13, 100%) | Abd/thoracic pain [77] | 54 | 8.1 [5–10] | 158 | Polidocanol | 15% (1, intracystic bleeding, procedure aborted) | 69% | 92 [3–100] | NR | 1 | 23% |
| Yu ( |
2006–2011 | RS | 7 | 45 (52, 68%) | Abd pain/fullness [78] | NR | 8.3±1.8 | 319 | Ethanol | 7% (1, transient pain) | 84% | NR | NR | 12 | 16% |
| Lee ( |
2009–2012 | RS | 5 | 17 (19, 88%) | Abd pain/fullness [65] | 26 | 8.9 [7–11] | 369 | Ethanol | 18% (2, transient fever treated with antibiotics) | 100% | 98 [97–100] | 5 [3–8] | 13 [8–22] | nil |
| Souftas ( |
NR | PS | 6 | 10 (14, 60%) | Discomfort [80] | 60 | 9.1 [7–13] | 408 | 15% saline solution and bleomycin | Nil | 100% | 100 | 1 | 12 | NR |
| Akhan ( |
1993–2012 | RS | 5 | 35 (39, 88%) | Abd pain [80] | NR | NR | 94 | Ethanol | 3% (3A, liver abscess, treated with aspirated) | 100% | 95 [60–100] | 1 | 38 [4–173] | nil |
| Larssen ( |
1993–2010 | RS | 4 | 47 (51, 89%) | NR | NR | NR | 520 | Ethanol | 2% (2, ethanol intoxication) | NR | 99 [83–100] | NR | 56 [24–193] | nil |
| Jang ( |
2003–2013 | RS | 6 | 42 (43, 21%) | Abd pain/fullness [81] | 76 | 12 [6–21] | 930 | Ethanol | 19% (1, pain during ethanol injection, procedure aborted) | 94% | 95 | NR | 33 [12–106] | NR |
| Wijnands ( |
2003–2014 | PS/RS | 6 | 86 (NR, 62%) | NR | 70 | 11 [9–15] | 696 | Ethanol | 11% (2, cyst infection treated with antibiotics) | 89% | 94 | NR | NR | NR |
| Danza ( |
2004/2015 | RS | 6 | 21 (24, 86%) | Abd fullness [87] | NR | NR | NR | Minocycline, hydrochloride | 12% (2, intracystic hemorrhage) | 100% | NR | NR | NR [24–120] | nil |
| Wijnands ( |
2014–2016 | RCT | 6 | 34 (NR, 32%) | Abd pain [80] | NR | 9.7 | 450 | Ethanol | 9% (3A, cyst re-aspiration due to symptoms persistency) | 90% | NR | NR | 6 | NR |
NOS, Newcastle Ottawa Scale; SC, solitary cyst; D-C, Dindo-Clavien; LOS, length of stay; SC, solitary cyst; MC, multiple cyst; RS, retrospective study; NR, not reported; abd, abdominal.
In the group of articles reporting on LF for SN-SLC, operation duration was reported by 26 studies and accounted for a mean of 96 minutes (50 to 200 minutes). Overall, conversion rate was reported by 32 articles and 19 (2.2%) patients needed for a conversion to laparotomy during LF. Postoperative morbidity rate was reported by all included study on LF, with 78 patients out of 1,058 (7.4%) experiencing a complication following surgery. Complication rate was nil in 16 (39%) studies. Among 38 (93%) studies reporting on Dindo-Clavien grade of postoperative complication, at least one major complication was observed in 34% of studies, mortality being nil, and among 37 studies reporting the duration of postoperative recovery, the average length of hospital stay following LF was 4 days (0 to 9 days). Concerning PS studies, of 20 (out of 22, 91%) studies reporting on postoperative complications, 52 patients (9.3%) experienced a postoperative complication. In two (10%) studies no complications occurred following PS while in the remaining studies the most serious postoperative complication was graded as minor and major in 15 (75%) and 3 (15%) studies, respectively. Among 3 studies reporting postoperative major complications, one death due to acetic acid intoxication occurred, which represented the only death in the overall study population.
The median duration of follow-up following treatment was 43 months (10 to 77 months) and 24 months (1 to 56 months) among LF and PS articles, respectively. Among 40 LF studies (97%) analyzing long-term outcomes, the overall rate of symptoms recurrence requiring an operative management was 6% (63 out 1,043 patients) and was nil in 12 studies. Among 6 studies specifying if the symptoms recurrence occurred among patients affected by solitary versus multiple SN-SLC, of 17 patients undergoing an additional treatment (out of 218, 7.8%), 88% occurred among patients affected by multiple SN-SLC. Among 22 PS studies reporting on long-term outcomes, the overall rate of symptoms recurrence requiring an operative management was 9.7% (27 out 278 patients) and was nil in 5 studies.
Discussion
In the current review, we retrospectively analyzed and compared clinical and surgical characteristics and perioperative and long-term outcomes of 1,678 patients from 62 studies published up to August 2019 and reporting on LF and/or PS for patients affected by SN-SLC. Despite the choice of the correct treatment option for SN-SLC still represents a matter of debate, no trial comparing LF and PS have been performed and only studies comparing LF and PS in the management of SN-SLC report either on small groups of patients (37) or on outdated data referring to patients treated before 2000 (73).
According to our inclusion criteria, the number of studies reporting on LF was higher, compared to PS studies, and was paralleled by higher number of patients treated with LF, compared to PS, however comparison between studies reporting on patients treated with LF versus PS showed overall similarity in terms of study quality and risk of bias (according to means of NOS scores), of median number of patients included in the singular studies and of symptoms at the time of SN-SLC diagnosis. Cysts treated with LF were larger and more often located in the left liver, compared to cysts treated with PS. This probably reflects the trend to recommend a more invasive (and hypothetically effective) treatment to patients affected by larger cysts, as well as the relative difficulty to treat with LF cysts located in the right liver, mainly in the posterior sector.
Concerning treatment safety, despite overall rate of postoperative complication was similar between patients undergoing LF and PS, among studies reporting Dindo-Clavien grade of post-procedure complication, at least one major complication was more often observed after LF, compared to PS. Following cyst treatment, studies focusing on LF, compared to those focusing on PS, reported a slightly lower rate of cyst recurrence requiring for additional treatment. Such results parallel recommendations from ACG and EFSUMB guidelines (5,6), suggesting PS as a good alternative to LF, with lower rates of serious post-procedure complication but lower efficacy of PS, compared to LF, while reflecting different mechanisms for symptoms relief and recurrence following cyst treatment.
When considering LF, the aspiration of the cyst and the removal of its wall determine a rapid symptom relief, while an incomplete deroofing or the development of a false lumen due to adhesions with close anatomical structures and tissues are considered the main responsible for cyst recurrence (39,74). In contrast, in order to correctly report and quantify efficacy results of PS is important to know that, following evacuation of the cyst using aspiration sclerotherapy, cyst fluid will reaccumulate within some days and will subsequently and slowly disappear after at least 5 to 6 months (22), highlighting the importance of a long follow-up duration to adequately assess efficacy of PS. In this context, the average follow-up duration among PS, almost half than that of studies focusing on LF studies, may be responsible for a partial underestimation of the real rate of symptomatic recurrence following PS and as such should be carefully evaluated.
The current review has some limitations: first is the heterogeneity of outcomes following treatment, among LF and PS studies, which probably accounts for different study population, given the presence in the studies analyzed of a variable number of patients affected by multiple, other than single SN-SLC. Thus, it may be hypothesized that differences in rates of symptoms recurrence requiring additional treatment may be related to different rates of patients affected by multiple cysts among studies. Among strengths of the study, we highlight that it is based on an up-to-date Medline search, with an independent screening of found references, as well as of risk of bias assessment of finally included studies, performed by two authors (Giuseppe Zimmitti and V Sega). Exclusion of studies focused on polycystic liver disease, namely characterized by a different natural history and by different outcomes and management, as well as of studies including less than five patients and of duplicated datasets, contributed to reduce biases in reported outcomes rates.
In conclusion, both PS and LF are widely used for the treatment of SN-SLC and are both associated with high safety and efficacy. Despite a moderate heterogeneity in outcome results among two groups of papers analyzed, PS seems to be associated, compared to LF, with a lower rate of serious post-procedure complications and a higher risk of symptomatic cyst recurrence. However, heterogeneity in study groups makes outcome results comparison between PS and LF difficult and prevents from stating definitive conclusion.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Francesco Ardito) for the series “The Role of Minimally Invasive Liver Surgery to Treat Hepatic Benign Disease” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2020.03.05). The series “The Role of Minimally Invasive Liver Surgery to Treat Hepatic Benign Disease” was commissioned by the editorial office without any funding or sponsorship. ER serves as an unpaid editorial board member of Laparoscopic Surgery from October 2019 to September 2021. MAH serves as an unpaid editorial board member of Laparoscopic Surgery from June 2019 to May 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lantinga MA, Gevers TJ, Drenth JP. Evaluation of hepatic cystic lesions. World J Gastroenterol 2013;19:3543-54. [Crossref] [PubMed]
- Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol 1989;62:335-7. [Crossref] [PubMed]
- Sanchez H, Gagner M, Rossi RL, et al. Surgical management of non-parasitic cystic disease of the liver. Am J Surg 1991;161:113. [Crossref] [PubMed]
- Wijnands TF, Neijenhuis MK, Kievit W, et al. Evaluating health-related quality of life in patients with polycystic liver disease and determining the impact of symptoms and liver volume. Liver Int 2014;34:1578-83. [Crossref] [PubMed]
- Marrero JA, Ahn J, Rajender Reddy K, et al. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 2014;109:1328-47; quiz 1348. [Crossref] [PubMed]
- Dietrich CF, Lorentzen T, Appelbaum L, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III - Abdominal Treatment Procedures (Short Version). Ultraschall Med 2016;37:27-45. [Crossref] [PubMed]
- Mahid SS, Hornung CA, Minor KS, et al. Systematic reviews and meta-analysis for the surgeon scientist. Br J Surg 2006;93:1315-24. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available online: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- Martin IJ, McKinley AJ, Currie EJ, et al. Tailoring the management of nonparasitic liver cysts. Ann Surg 1998;228:167-72. [Crossref] [PubMed]
- Diez J, Decoud J, Gutierrez L, et al. Laparoscopic treatment of symptomatic cysts of the liver. Br J Surg 1998;85:25-7. [Crossref] [PubMed]
- Zacherl J, Scheuba C, Ihnhof M, et al. Long-term results after laparoscopic unroofing congenital liver cysts. Surg Endosc 2000;14:59-62. [Crossref] [PubMed]
- Katkhouda N, Mavor E, Gugenheim J, et al. Laparoscopic management of benign cystic lesions of the liver. J Hepatobiliary Pancreat Surg 2000;7:212-7. [Crossref] [PubMed]
- Hansman MF, Ryan JAJ, Holmes JH, et al. Management and longterm follow-up of hepatic cysts. Am J Surg 2001;181:404-10. [Crossref] [PubMed]
- Gigot JF, Metairie S, Etienne J, et al. The surgical management of congenital liver cysts. Surg Endosc 2001;15:357-63. [Crossref] [PubMed]
- Regev A, Reddy KR, Berho M, et al. Large Cystic Lesions of the Liver in Adults: A 15-year Experience in a Tertiary center. J Am Coll Surg 2001;193:36-45. [Crossref] [PubMed]
- Schachter P, Sorin V, Avni Y, et al. The role of laparoscopic ultrasound in the minimally invasive management of symptomatic hepatic cysts. Surg Endosc 2001;15:364-7. [Crossref] [PubMed]
- Tocchi A, Mazzoni G, Costa G, et al. Symptomatic nonparasitic hepatic cysts: options for and results of surgical management. Arch Surg 2002;137:154-8. [Crossref] [PubMed]
- Tan YM, Ooi LL, Soo KC, et al. Does laparoscopic fenestration provide long-term alleviation for symptomatic cystic disease of the liver? ANZ J Surg 2002;72:743-5. [Crossref] [PubMed]
- Petri A, Hohn J, Makula E, et al. Experience with different methods of treatment of nonparasitic liver cysts. Langenbecks Arch Surg 2002;387:229-33. [Crossref] [PubMed]
- Tagaya N, Nemoto T, Kubota K. Long-Term Results of Laparoscopic Unroofing of Symptomatic Solitary Nonparasitic Hepatic Cysts. Surg Laparosc Endosc Percutan Tech 2003;13:76-9. [Crossref] [PubMed]
- Fiamingo P, Tedeschi U, Veroux M, et al. Laparoscopic treatment of simple hepatic cysts and polycystic liver disease. Surg Endosc 2003;17:623-6. [Crossref] [PubMed]
- Hsu KL, Chou FF, Ko SF, et al. Laparoscopic Fenestration of Symptomatic Liver Cysts. Surg Laparosc Endosc Percutan Tech 2005;15:66-9. [Crossref] [PubMed]
- Fabiani P, Iannelli A, Chevallier P, et al. Long-term outcome after laparoscopic fenestration of symptomatic simple cysts of the liver. Br J Surg 2005;92:596-7. [Crossref] [PubMed]
- Neri V, Ambrosi A, Fersini A, et al. Laparoscopic treatment of biliary hepatic cysts: short- and medium-term results. HPB 2006;8:306-10. [Crossref] [PubMed]
- Palanivelu C, Jani K, Malladi V. Laparoscopic management of benign nonparasitic hepatic cysts: a prospective nonrandomized study. South Med J 2006;99:1063-7. [Crossref] [PubMed]
- Szabó LS, Takács I, Arkosy P, et al. Laparoscopic treatment of nonparasitic hepatic cysts. Surg Endosc 2006;20:595-7. [Crossref] [PubMed]
- Bai XL, Liang TB, Yu J, et al. Long-term results of laparoscopic fenestration for patients with congenital liver cysts. Hepatobiliary Pancreat Dis Int 2007;6:600-3. [PubMed]
- Gall TM, Oniscu GC, Madhavan K, et al. Surgical management and longterm follow-up of non-parasitic hepatic cysts. HPB 2009;11:235-41. [Crossref] [PubMed]
- Gamblin TC, Holloway SE, Heckman JT, et al. Laparoscopic resection of benign hepatic cysts: a new standard. J Am Coll Surg 2008;207:731-6. [Crossref] [PubMed]
- Mazza OM, Fernandez DL, Pekolj J, et al. Management of Nonparasitic Hepatic Cysts. J Am Coll Surg 2009;209:733-9. [Crossref] [PubMed]
- Treckmann JW, Paul A, Sgourakis G, et al. Surgical treatment of nonparasitic cysts of the liver: open versus laparoscopic treatment. Am J Surg 2010;199:776-81. [Crossref] [PubMed]
- Loehe F, Globke B, Marnoto R, et al. Long-term results after surgical treatment of nonparasitic hepatic cysts. Am J Surg 2010;200:23-31. [Crossref] [PubMed]
- Donati M, Stavrou GA, Wellman A. Laparoscopic deroofing of hepatic cysts: The most effective treatment option. Clin Ter 2010;161:345-8. [PubMed]
- Faulds JM, Scudamore CH. Technical report of a novel surgical technique: laparoscopic cyst fenestration and falciform ligament pedicle graft for treatment of symptomatic simple hepatic cysts. J Laparoendosc Adv Surg Tech A 2010;20:857-61. [Crossref] [PubMed]
- Kim SR, Lee DS, Park IY. Managements of simple liver cysts: ablation therapy versus cyst unroofing. Korean J Hepatobiliary Pancreat Surg 2012;16:134-7. [Crossref] [PubMed]
- Wahba R, Kleinert R, Prenzel K, et al. Laparoscopic deroofing of nonparasitic liver cysts with or without greater omentum flap. Surg Laparosc Endosc Percutan Tech 2011;21:54-8. [Crossref] [PubMed]
- Mazoch MJ, Dabbous H, Shokouh-Amiri H, et al. Management of giant liver cysts. J Surg Res 2011;167:e125-30. [Crossref] [PubMed]
- Kamphues C, Rather M, Engel S, et al. Laparoscopic fenestration of non-parasitic liver cysts and health-related quality of life assessment. Updates Surg 2011;63:243-7. [Crossref] [PubMed]
- Scheuerlein H, Rauchfuss F, Franke J, et al. Clinical symptoms and sonographic follow-up after surgical treatment of nonparasitic liver cysts. BMC Surg 2013;13:42. [Crossref] [PubMed]
- Ardito F, Bianco G, Vellone M, et al. Long-term outcome after laparoscopic fenestration of simple liver cysts. Surg Endosc 2013;27:4670-4. [Crossref] [PubMed]
- Gocho T, Misawa T, Haruki K, et al. Transumbilical single-incision laparoscopic deroofing for hepatic and splenic cysts. Surg Endosc 2013;29:S485.
- Noerregaard CL, Ainswort AP. Good results after laparoscopic marsupialization of simple liver cysts. Dan Med J 2014;61:A4866. [PubMed]
- Lee DH, Cho JY, Han HS, et al. Laparoscopic treatment of hepatic cysts located in the posterosuperior segments of the liver. Ann Surg Treat Res 2014;86:232-6. [Crossref] [PubMed]
- Wu S, Li Y, Tian Y, et al. Single-incision laparoscopic surgery versus standard laparoscopic surgery for unroofing of hepatic cysts. JSLS 2014;18:246-51. [Crossref] [PubMed]
- Manterola C, Otzen T. MINCIR Group. Laparoscopic Surgery in Nonparasitic Cysts of the Liver: Results Observed in a Series of Consecutive Cases. Surg Laparosc Endosc Percutan Tech 2016;26:308-12. [Crossref] [PubMed]
- Debs T, Kassir R, Reccia I, et al. Technical challenges in treating recurrent non-parasitic hepatic cysts. Int J Surg 2016;25:44-8. [Crossref] [PubMed]
- Kisiel A, Vass DG, Navarro A, et al. Longterm patient-reported outcomes after laparoscopic fenestration of symptomatic liver cysts. Surg Laparosc Endosc Percutan Tech 2017;27:e80-2. [Crossref] [PubMed]
- de Reuver P, van der Walt I, Albania M, et al. Long-term outcomes and quality of life after surgical or conservative treatment of benign simple liver cysts. Surg Endosc 2018;32:105-13. [Crossref] [PubMed]
- Tsirlis T, Thakkar R, Sen G, et al. Robotic fenestration of massive liver cysts using EndoWrist technology. Int J Med Robot 2019;15:e1994 [Crossref] [PubMed]
- Kairaluoma MI, Leinonen A, Ståhlberg M, et al. Percutaneous aspiration and alcohol sclerotherapy for symptomatic hepatic cysts. An alternative to surgical intervention. Ann Surg 1989;210:208-15. [Crossref] [PubMed]
- Montorsi M, Torzilli G, Fumagalli U, et al. Percutaneous alcohol sclerotherapy of simple hepatic cysts. Results from a multicentre survey in Italy. HPB Surg 1994;8:89-94. [Crossref] [PubMed]
- Tikkakoski T, Mäkelä JT, Leinonen S, et al. Treatment of symptomatic congenital hepatic cysts with single-session percutaneous drainage and ethanol sclerosis: technique and outcome. J Vasc Interv Radiol 1996;7:235-9. [Crossref] [PubMed]
- Cellier C, Cuenod CA, Deslandes P, et al. Symptomatic hepatic cysts: treatment with single-shot injection of minocycline hydrochloride. Radiology 1998;206:205-9. [Crossref] [PubMed]
- Poźniczek M, Wysocki A, Bobrzyński A, et al. Sclerosant therapy as first-line treatment for solitary liver cysts. Dig Surg 2004;21:452-4. [Crossref] [PubMed]
- Yang CF, Liang HL, Pan HB, et al. Single-session prolonged alcohol-retention sclerotherapy for large hepatic cysts. AJR Am J Roentgenol 2006;187:940-3. [Crossref] [PubMed]
- Erdogan D, van Delden OM, Rauws EA, et al. Results of percutaneous sclerotherapy and surgical treatment in patients with symptomatic simple liver cysts and polycystic liver disease. World J Gastroenterol 2007;13:3095-100. [Crossref] [PubMed]
- Zerem E, Imamović G, Omerović S. Percutaneous treatment of symptomatic non-parasitic benign liver cysts: single-session alcohol sclerotherapy versus prolonged catheter drainage with negative pressure. Eur Radiol 2008;18:400-6. Erratum in: Eur Radiol 2008 Feb;18(2):407. [Crossref] [PubMed]
- Nakaoka R, Das K, Kudo M, et al. Percutaneous aspiration and ethanolamine oleate sclerotherapy for sustained resolution of symptomatic polycystic liver disease: an initial experience. AJR Am J Roentgenol 2009;193:1540-5. [Crossref] [PubMed]
- Jusufovic R, Zerem E. Percutaneous treatment of symptomatic non-parasitic benign liver cysts with 20% NaCl solution. Med Arh 2011;65:35-7. [PubMed]
- Yan-Hong F, Lin-Xue Q, Hai-Ma G, et al. Sclerotherapy of simple hepatic cysts by repeated aspiration and alcohol instillation. Turk J Gastroenterol 2012;23:359-65. [Crossref] [PubMed]
- Spârchez Z, Radu P, Zaharie F, et al. Percutaneous treatment of symptomatic non-parasitic hepatic cysts. Initial experience with single-session sclerotherapy with polidocanol. Med Ultrason 2014;16:222-8. [PubMed]
- Yu JH, Du Y, Li Y, et al. CT-guided sclerotherapy for simple renal cysts: value of ethanol concentration monitoring. Korean J Radiol 2014;15:80-6. [Crossref] [PubMed]
- Lee S, Seo DW, Paik WH, et al. Ethanol lavage of huge hepatic cysts by using EUS guidance and a percutaneous approach. Gastrointest Endosc 2014;80:1014-21. [Crossref] [PubMed]
- Souftas VD, Kosmidou M, Karanikas M, et al. Symptomatic abdominal simple cysts: is percutaneous sclerotherapy with hypertonic saline and bleomycin a treatment option? Gastroenterol Res Pract 2015;2015:489363 [Crossref] [PubMed]
- Akhan O, Islim F, Balci S, et al. Percutaneous Treatment of Simple Hepatic Cysts: The Long-Term Results of PAIR and Catheterization Techniques as Single-Session Procedures. Cardiovasc Intervent Radiol 2016;39:902-8. [Crossref] [PubMed]
- Larssen TB, Viste A, Horn A, et al. Single-session alcohol sclerotherapy of symptomatic liver cysts using 10–20 min of ethanol exposure: no recurrence at 2–16 years of follow-up. Abdom Radiol (NY) 2016;41:1776-81. [Crossref] [PubMed]
- Jang SY, Park SY, Tak WY, et al. Long-term follow-up of large symptomatic hepatic cysts treated by percutaneous ethanol sclerotherapy. Acta Radiol 2016;57:1205-9. [Crossref] [PubMed]
- Wijnands TF, Ronot M, Gevers TJ, et al. Predictors of treatment response following aspiration sclerotherapy of hepatic cysts: an international pooled analysis of individual patient data. Eur Radiol 2017;27:741-8. [Crossref] [PubMed]
- Danza FM, Falcione M, Bordonaro V, et al. Minocycline hydrochloride as a soft sclerotizing agent for symptomatic simple renal and hepatic cysts. Eur Rev Med Pharmacol Sci 2017;21:408-15. [PubMed]
- Wijnands TFM, Gevers TJG, Lantinga MA, et al. Pasireotide does not improve efficacy of aspiration sclerotherapy in patients with large hepatic cysts, a randomized controlled trial. Eur Radiol 2018;28:2682-9. [Crossref] [PubMed]
- Moorthy K, Mihssin N, Houghton PW. The management of simple hepatic cysts: sclerotherapy or laparoscopic fenestration. Ann R Coll Surg Engl 2001;83:409-14. [PubMed]
- Emmermann A, Zornig C, Lloyd DM, et al. Laparoscopic treatment of nonparasitic cysts of the liver with omental transposition flap. Surg Endosc 1997;11:734-6. [Crossref] [PubMed]
Cite this article as: Zimmitti G, Sega V, Rosso E, Treppiedi E, Manzoni A, Codignola C, Garatti M, Abu Hilal M. Systematic review of laparoscopic fenestration and percutaneous sclerotherapy in the management of symptomatic nonparasitic simple liver cysts. Laparosc Surg 2020;4:44.