
An advanced intrahepatic cholangiocarcinoma treated with minimally invasive resection: case report
Introduction
Since the first report of laparoscopic liver resection (LLR) in 1991 (1), accumulated experiences and technological developments have facilitated the expansion of the indication (2-4). It is becoming clear that the magnified caudal view offered by laparoscopy allows improved visualization, especially for the hilar and dorsal area of the liver, and is thus beneficial for the dissection of hilar Glissonian pedicles and the inferior vena cava (IVC) (4-6). LLRs of major hepatectomy and, even, with combined resection of major hepatic veins are now increasingly reported (7-9).
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver malignancy with poor prognosis (10-12). The incidence of ICC has been increasing around the world (13) and surgical resection is still the only cure. Lymph node (LN) metastasis is the most important prognostic factors after surgery (14-16). Although the importance of LN dissection is under discussion, several studies suggested the necessity of LN dissection for survival benefits (17,18). Furthermore, ICC often has the need for bile duct reconstruction due to its biliary/peri-biliary invasion (10,11). Although reports for LLR on ICC are increasing (19-23), its major challenges are in the difficulties of LN dissection and bile duct reconstruction (22,24).
Here we present a case report of advanced ICC, who underwent unique combined minimally invasive surgery of LLR and LN dissection and bile duct reconstruction through a small laparotomy and survived 6.5 years in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/ls-2020-mirlm-04).
Case presentation
A 70-year-old man with 9.5 cm mass and bile duct dilatation on the right liver was referred to our department for treatment of the lesion. These had been revealed by the abdominal ultrasonography undertaken for his liver dysfunction and body weight loss. The patient had only the history of medication for his diabetes mellitus, but not the other past and family histories including liver related disorders.
The laboratory data showed decreased plasma albumin level (3.3 g/dL) and elevations in plasma levels of aspartate transaminase (67 IU/L), alanine transaminase (77 IU/L), alkaline phosphatase (3,001 U/L), gamma-glutamyl transpeptidase (825 U/L), fibrinogen (582 mg/dL), C-reactive protein (0.84 mg/dL), hemoglobin A1c (10.5%) and indocyanine green retension rate at 15 minutes (11.2%), though the other data were all within normal range including plasma bilirubin level (0.5 mg/dL), prothrombin time (1.01, INR) and platelet count (218,000/microL). The tumor markers showed elevations (CEA 5.4 ng/mL, CA19-9 149.8 U/mL). Neither hepatitis B nor C virus-related markers are positive.
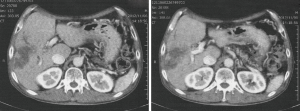
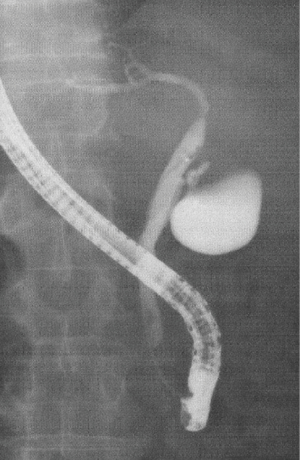
The contrast enhanced abdominal computed tomography (CT) demonstrated 9.5-cm-sized low-density lesions in the right liver with the dilatation of right intrahepatic bile duct (Figure 1). The findings of endoscopic retrograde cholangiography showed obstruction of the right branch of bile duct and stenosis on hilar portion of the main duct (common hepatic duct) (Figure 2).
Combined minimally invasive surgery of LLR and LN dissection and bile duct reconstruction through a small laparotomy was planned under the diagnosis of advanced ICC with the invasion to common hepatic duct.
Surgical procedures
During the surgery, the patient was placed in a supine position.
The 8-cm caudal-cranial incision on the right upper abdomen was placed just above the hepatoduodenal ligament under the guide of extracorporeal ultrasonography. Through this incision (Figure 3A), LN dissection of the areas of hilar plate, hepatoduodenal ligament, common hepatic artery and upper dorsal area of pancreas head and, also, divisions of right hepatic artery and portal vein were performed. Subsequently, bile duct divisions, at the line of right-side edge of umbilical portal vein in proximal (with negative findings of carcinoma invasion on frozen section pathological examination) and the upper margin of pancreas head in distal, were also performed through the laparotomy.
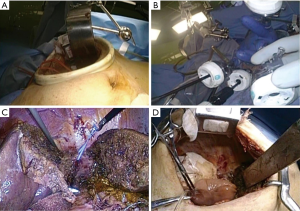
After inserting a wound protector devise with a surgical glove to the incision of laparotomy, the 5 trocar ports of 12-mm-size were introduced, 2 from glove fingers and 3 from new incisions surrounding the first incision (Figure 3B). CO2-pneumoperitonium (8–12 mmHg) was established through the ports. The Pringle maneuver was not applied to this patient, since the liver parenchymal transection was following after hilar lymph nodal dissection and divisions of right hepatic artery and portal vein. Transection of the liver parenchyma was performed on the Rex-Cantlie line, which was confirmed as the ischemic demarcation line, exposing IVC in the laparoscopic specific caudal approach without prior mobilization of the liver (Figure 3C). After the completion of the transection, the root of right hepatic vein was divided with laparoscopic stapler and the resected liver was dissected and removed laparoscopically.
After the completion of liver resection, bile duct reconstruction with Roux-en-Y hepatico-jejunostomy was performed using single-layer interrupted suture with 5-0 absorbable monofilament materials through the small laparotomy (Figure 3D). A surgical drain was placed in right subphrenic space. The operation took 545 min and the estimated intraoperative blood loss was 847 mL with 4 units of red blood cell transfusion.
Pathological examination of the tumor identified it to be well-differentiated tubular adenocarcinoma (ICC) with invasions to the confluence of the right hepatic duct to the common hepatic duct and right portal vein, there was no evidence of metastasis in 11 LNs dissected as surgical specimen.
The patient recovered uneventfully and discharged at 14 days after the surgery. He survived for 6.5 years with the recurrence at 4 years after the surgery and died of the disease.
Written informed consent was obtained from the patient for the treatment process and publication of this study and any accompanying images.
Discussion
The first report of LLR for ICC (left lateral sectionectomy with LN dissection) was in 2002 (25). Thereafter, more challenging laparoscopic left hepatectomy, caudate hepatectomy, and right hepatectomy have been reported (26,27). A case series report of 11 LLRs for ICC was in 2015 (19). Recently, Wei et al. demonstrated that LLR for ICC has similar feasibility and safety in the comparison to open procedure by analyzing 384 and 2,147 patients who had undergone LLR and open procedure in their meta-analysis (23). They described that the rate of R0 resection in LLR patients was higher than open (81.6% versus 73.8%) and mentioned that laparoscopic magnified vision allowed the resection of lesions and hepatectomies safe and efficient.
Although reports of LLR for ICC are increasing as above, there are still challenges in the procedure, the difficulties of LN dissection and bile duct reconstruction (22,24). The incidence of LN metastases from ICC is high and ICC often has the need for bile duct resection/reconstruction like present case due to its biliary/peri-biliary invasion. The techniques for LN dissection have been applied in other established procedures (28,29). Although dissection of the posterosuperior area of pancreas head is difficult, it is reported that this dissection can be easier with the Kocher maneuver (30,31). However, laparoscopic bile duct reconstruction are still demanding techniques with small number of reports (31-34).
In present case, we choose the minimally invasive combination of laparoscopic liver transection and mobilization plus LN dissection and bile duct reconstruction through small laparotomy. There are several reports of hybrid procedures, such as laparoscopic pancreaticoduodenectomy with reconstruction through mini-laparotomy for pancreatic tumors (35) and laparoscopic plus robotic (for reconstruction) hepatopancreaticoduodenectomy for cholangiocarcinoma (24). These hybrid procedures can make the complicated LN dissection and bile duct reconstruction easier. Furthermore, small laparotomy may facilitate the decision making for the dividing point of bile duct with its good overview for whole hepato-biliary system, contrary to the laparoscopic magnified but locally limited view.
Present case, who underwent minimally invasive combination surgery for advanced ICC, recovered uneventfully and survived for 6.5 years, although he developed recurrence at 4 years after the surgery and died of the disease. We believe this is one of the choices for advanced ICC, which has needs for LN dissection and bile duct reconstruction, especially in the early stage of the learning curve for the laparoscopic procedures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giammauro Berardi) for the series “Minimally Invasive Resections for Liver Malignancies: Among Certainties and Controversies” published in Laparoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/ls-2020-mirlm-04
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-2020-mirlm-04). The series “Minimally Invasive Resections for Liver Malignancies: Among Certainties and Controversies” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reich H, McGlynn F, DeCaprio J, et al. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956-8. [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Tsuchiya M, Otsuka Y, Tamura A, et al. Status of endoscopic liver surgery in Japan: a questionnaire survey conducted by the Japanese Endoscopic Liver Surgery Study Group. J Hepatobiliary Pancreat Surg 2009;16:405-9. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Tomishige H, Morise Z, Kawabe N, et al. Caudal approach to pure lparoscopic posterior sectionectomy under the laparoscopy-specific view. World J Gastrointest Surg 2013;5:173-7. [Crossref] [PubMed]
- Soubrane O, Schwarz L, Cauchy F, et al. A conceptual technique for laparoscopic right hepatectomy based on facts and oncologic principles: the caudal approach. Ann Surg 2015;261:1226-31. [Crossref] [PubMed]
- Morise Z, Kawabe N, Tomishige H, et al. How far can we go with laparoscopic liver resection for hepatocellular carcinoma? Laparoscopic sectionectomy of the liver combined with the resection of the major hepatic vein main trunk. Biomed Res Int 2015;2015:960752 [Crossref] [PubMed]
- Yoon YS, Han HS, Cho JY, et al. Laparoscopic liver resection for centrally located tumors close to the hilum, major hepatic veins, or inferior vena cava. Surgery 2013;153:502-9. [Crossref] [PubMed]
- Torzilli G, Donadon M, Marconi M, et al. Systematic extended right posterior sectionectomy: a safe and effective alternative to right hepatectomy. Ann Surg 2008;247:603-11. [Crossref] [PubMed]
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. [Crossref] [PubMed]
- Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261-80. [Crossref] [PubMed]
- Chan KM, Tsai CY, Yeh CN, et al. Characterization of intrahepatic cholangiocarcinoma after curative resection: outcome, prognostic factor, and recurrence. BMC Gastroenterology 2018;18:180. [Crossref] [PubMed]
- Antwi SO, Mousa OY, Patel T. Racial, Ethnic, and Age Disparities in Incidence and Survival of Intrahepatic Cholangiocarcinoma in the United States; 1995-2014. Ann Hepatol 2018;17:274-85. [Crossref] [PubMed]
- Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048-56. [Crossref] [PubMed]
- Uchiyama K, Yamamoto M, Yamaue H, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2011;18:443-52. [Crossref] [PubMed]
- Lurje G, Bednarsch J, Czigany Z, et al. The prognostic role of lymphovascular invasion and lymph node metastasis in perihilar and intrahepatic cholangiocarcinoma. Eur J Surg Oncol 2019;45:1468-78. [Crossref] [PubMed]
- Zhang XF, Chakedis J, Bagante F, et al. Trends in use of lymphadenectomy in surgery with curative intent for intrahepatic cholangiocarcinoma. Br J Surg 2018;105:857-66. [Crossref] [PubMed]
- Kim SH, Han DH, Choi GH, et al. Oncologic Impact of Lymph Node Dissection for Intrahepatic Cholangiocarcinoma: a Propensity Score-Matched Study. J Gastrointest Surg 2019;23:538-44. [Crossref] [PubMed]
- Uy BJ, Han HS, Yoon YS, et al. Laparoscopic Liver Resection for Intrahepatic Cholangiocarcinoma. J Laparoendosc Adv Surg Tech A 2015;25:272-7. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma: a propensity score-based case-matched analysis from a single institution. Surg Endosc 2016;30:1999-2010. [Crossref] [PubMed]
- Lee W, Park JH, Kim JY, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 2016;30:4835-40. [Crossref] [PubMed]
- Wei F, Lu C, Cai L, et al. Can laparoscopic liver resection provide a favorable option for patients with large or multiple intrahepatic cholangiocarcinomas? Surg Endosc 2017;31:3646-55. [Crossref] [PubMed]
- Wei F, Wang G, Ding J, et al. Is It Time to Consider Laparoscopic Hepatectomy for Intrahepatic Cholangiocarcinoma? A Meta-Analysis. J Gastrointest Surg 2020;24:2244-50. [Crossref] [PubMed]
- Chong EH, Choi SH. Hybrid Laparoscopic and Robotic Hepatopancreaticoduodenectomy for Cholangiocarcinoma. J Gastrointest Surg. 2019;23:1947-8. [Crossref] [PubMed]
- Harimoto N, Shimada M, Tsujita E, et al. Laparoscopic hepatectomy and dissection of lymph nodes for intrahepatic cholangiocarcinoma. Case report. Surg Endosc 2002;16:1806. [Crossref] [PubMed]
- Abu Hilal M, Badran A, Di Fabio F, et al. Pure Laparoscopic En Bloc Left Hemihepatectomy and Caudate Lobe Resection in Patients with Intrahepatic Cholangiocarcinoma. J Laparoendosc Adv Surg Tech A 2011;21:845-9. [Crossref] [PubMed]
- Takahashi M, Wakabayashi G, Nitta H, et al. Pure laparoscopic right hepatectomy by anterior approach with hanging maneuver for large intrahepatic cholangiocarcinoma. Surg Endosc 2013;27:4732-3. [Crossref] [PubMed]
- Cai J, Wei D, Cao CF, et al. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg 2011;28:331-7. [Crossref] [PubMed]
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. [Crossref] [PubMed]
- Itano O, Oshima G, Minagawa T, et al. Novel strategy for laparoscopic treatment of pT2 gallbladder carcinoma. Surg Endosc 2015;29:3600-7. [Crossref] [PubMed]
- Shirobe T, Maruyama S. Laparoscopic radical cholecystectomy with lymph node dissection for gallbladder carcinoma. Surg Endosc 2015;29:2244-50. [Crossref] [PubMed]
- Gumbs AA, Hoffman JP. Laparoscopic radical cholecystectomy and Roux-en-Y choledochojejunostomy for gallbladder cancer. Surg Endosc 2010;24:1766-8. [Crossref] [PubMed]
- Asbun HJ, Harada E, Stauffer JA. Tips for laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2016;23:E5-9. [Crossref] [PubMed]
- Abbas HM, Yassin NA, Ammori BJ. Laparoscopic resection of type I choledochal cyst in an adult and Roux-en-Y hepaticojejunostomy: a case report and literature review. Surg Laparosc Endosc Percutan Tech 2006;16:439-44. [Crossref] [PubMed]
- Nakamura Y, Matsumoto S, Yoshioka M, et al. Successful laparoscopic pancreaticoduodenectomy for intraductal papillary mucinous neoplasm: a case report and a reliable technique for pancreaticojejunostomy. J Nippon Med Sch 2012;79:218-22. [Crossref] [PubMed]
Cite this article as: Morise Z. An advanced intrahepatic cholangiocarcinoma treated with minimally invasive resection: case report. Laparosc Surg 2020;4:45.


