
Does laparoscopic liver resection provide better outcomes than thermoablation in patients with hepatocellular carcinoma?—A systematic review
Introduction
The current EASL/AASLD guidelines indicate that liver transplantation, hepatic resection (HR) and percutaneous tumor ablation (TA) should be considered as curative therapies for hepatocellular carcinoma (HCC) (1). Among these, liver transplantation is the ideal option at earlier stages, however, shortage of organ donors and the advanced age of patients at diagnosis limited the indications of this treatment (2-4). Cirrhotic patients with a single small-size nodule and good liver function can benefit by both HR and ablation therapies increasing the survival, but disease recurrence following either treatment remains a great problem (5-8). Furthermore, in the Literature, HR shows similar to or better survival rates than percutaneous TA, with lower recurrence rates but with higher postoperative complications rates (5-8). For this reason, the current EASL/AASLD guidelines indicates that TA should be considered the first-line treatment for very early HCC [single small (≤2 cm) nodule], while HR should be reserved for patients with failure or contraindications to TA (1). This treatment is generally performed percutaneously, thus individual factors such as the operator skilling, risk of bleeding and nodule localization influenced its results in terms of either total necrosis or local recurrences (9-11). So, for problematic or dangerous locations of HCC nodules, laparoscopic HR (LHR) seems to obtain good results showing lower morbidity rates than open HR (12-15). For these reasons, we decided that it is more interesting to compare the TA outcomes to LHR, rather than open HR, particularly in patients with compensated (i.e., Child A) liver cirrhosis.
Therefore, our review aimed to assess the long-term and short-term clinical outcomes of these two HCC treatments analyzing the relevant studies published in the literature, in order to provide better information on the treatment strategy for cirrhotic patients with HCC.
Methods
A systematic research of PubMed, Science Citation Index, and Embase databases was accomplished for articles published before August 2019 comparing the use of TA and LHR treatments for small HCC nodules. We identified English language articles using the keywords “laparoscopic hepatectomy AND hepatocellular carcinoma” and “radiofrequency ablation AND hepatocellular carcinoma” to obtain all studies useful for this review analysis. Manual cross-referencing was accomplished, and we also analyzed the reference lists of the included articles to identify further undetected studies. Case reports and abstracts were excluded.
The inclusion criteria for our review were: (I) definitive diagnosis of HCC according BCLC criteria; (II) original works from nonrandomized controlled trials or randomized controlled trials (RCTs) only for adult patients; (III) the procedures selected were LHR (including laparoscopic or laparoscopic-assisted liver resection or robotic-assisted liver resection) and TA (including laparoscopic or percutaneous TA (IV) clearly documented indications for TA and LHR; (V) the primary outcomes were the overall survival (OS) rate and local recurrences rate, and the secondary outcome was the postoperative complication rate; (VI) one of the outcomes described below must be report; (VII) for the studies published more than once, we use only the most recent and complete study.
The data were strictly extracted by two independent reviewers and they included first author, publication year, recruitment period country, study design, mean follow-up, inclusion criteria, mean age, sex, etiology of cirrhosis, HCC Child-Pugh class, number and size of tumors, and clinical outcomes: the short-term outcomes included postoperative complication rates while the long-term clinical outcomes included were OS rates and local recurrence rate.
The Newcastle-Ottawa Scale (NOS) for case–control studies was used for evaluating the quality of the methodology which has been used in each study (16).
The meta-analysis was performed using the RevMan software version 5.3 (The Nordic Cochrane Center, Cochrane Collaboration, and Copenhagen, Denmark). For this meta-analysis, two groups of patients were analyzed: the TA-treated group and the LHR-treated group. The odds ratio (OR) with the 95% confidence interval (CI) was calculated to compare the OS, local recurrences and complications rates between the two groups. For studies that did not provide the mortality data, the survival data were extracted from the Kaplan-Meier curves by the Engauge Digitizer v.4.1 software. A Chi-squared test was used to evaluate heterogeneity among the included studies: a P value inferior to 0.05 confirmed that a significant heterogeneity was present across the studies.
Results
A total of 4,468 studies were initially identified by searching the electronic databases and through the manual cross-referencing. On the basis of the inclusion and exclusion criteria, we identified nine nonrandomized comparative studies evaluating TA and LHR as the primary treatment for HCC (17-25). All these studies were not multicenter and evaluated the outcomes retrospectively, including 560 patients treated with TA and 358 patients submitted to LHR. There were no RCTs that compared LHR and TA for the treatment of HCC. The baseline characteristics of these patients are summarized in Table 1. In our analysis, six studies used percutaneous TA treatments (17-21,24), one study used multimodal TA treatments (percutaneous or surgical approach) (25) and the remaining two studies used laparoscopic TA (22,23). In addition, three studies were performed in Japan (17,18,25), two in China (19,21), one in Switzerland (20) and three in Italy (22-24). The largest study included 264 patients (23) and the smallest study included 40 patients (22). The characteristics of the nine studies used for this meta-analysis are shown in Table 1, while the demographics data of the patients included in each study are shown in Table 2.
Table 1
| Author | Publication year | Period | Country | Study design | Follow-up (months) | Inclusion criteria | NOS score |
|---|---|---|---|---|---|---|---|
| Casaccia | 2017 | 2005–2010 | Italy | Retrospective | LHR 44.7±21.3, TA 40.3±30.8 | Single lesion with size ≤65 mm or ≤3 lesions with size ≤45 mm | 8 |
| Di Sandro | 2019 | 2006–2016 | Italy | Retrospective + PSM | 33 median (IQR: 17–56) | Single lesion with size ≤3 cm | 8 |
| Harada | 2016 | 2008–2015 | Japan | Retrospective + PSM | 29.3 median (range: 0.3–89.2) | Single lesion with size ≤5.0 cm or ≤3 lesions with size ≤3 cm; HCC with PH | 7 |
| Ito | 2016 | 2011–2013 | Japan | Retrospective + PSM | LHR: 21 median (range: 2–47), TA: 23 median (range: 4–44) | Surface HCC; 1–3 lesions with size ≤3 cm | 7 |
| Lai | 2016 | 2005–2010 | China | Retrospective | ≥33 years | Single lesion with size ≤5.0 cm or ≤3 lesions with size ≤3 cm | 7 |
| Santambrogio | 2018 | 1998–2017 | Italy | Retrospective | LHR 41.7±31.5, TA 38.7±32.3 | Single lesion with size ≤3 cm | 8 |
| Song | 2016 | 2007–2013 | China | Retrospective | 31.2 (21.1–49.5) | Single lesion with size <4 cm | 8 |
| Vitali | 2016 | 1998–2012 | Switzerland | Retrospective | 26 median (range: 2–129) | Single lesion with size ≤3 cm | 8 |
| Yamashita | 2019 | 2000–2016 | Japan | Retrospective | LHR: 37 mean (range: 1–160), TA: 56 mean (range: 2–163) | Single lesion with size ≤5.0 cm or ≤3 lesions with size ≤3 cm | 8 |
PSM, propensity score matching; IQR, interquartile range; TA, thermo-ablation; LHR, laparoscopic hepatic resection; NOS, Newcastle-Ottawa Scale.
Table 2
| Author | Age (mean ± SD) | Sex M/F (%) | HBV/HCV (%) | Cirrhosis (%) | Child A (%) | Single HCC (%) | HCC size (mm) |
|---|---|---|---|---|---|---|---|
| Casaccia | LHR: 64±9, TA: 61±7 | 74/26 | 30/41 | 100 | 78 | 56 | LHR: 33±14, TA: 26±13 |
| Di Sandro | LHR: 68 [62–76]*, TA: 67 [56–76]* | 70/30 | 18/59 | 91 | 100 | 100 | LHR: 25 [20–30]°, TA: 22 [18–30]° |
| Harada | LHR: 74±6, TA: 73±9 | 50/50 | 8/72 | 100 | LHR: 5.2±0.4§, TA: 5.5±0.7§ | NA | LHR: 18±6, TA: 16±6 |
| Ito | LHR: 67 [65–70]*, TA: 71 [69–74]* | 61/39 | 100 | 100 | 94 | 83 | LHR: 20 [18–21]*, TA: 17 [16–19]* |
| Lai | LHR: 56±13, TA: 63±11 | 87/13 | 79/3 | 72 | 93 | 92 | LHR: 30±11, TA: 24±9 |
| Santambrogio | LHR: 68±9, TA: 69±9 | 73/27 | 14/68 | 100 | 100 | 100 | LHR: 21±7, TA: 19±6 |
| Song | LHR: 48±10, TA: 48±11 | 90/10 | 96/NA | 79 | 99 | 100 | ≤2: 47% |
| Vitali | LHR: 61 [31–84]*, TA: 67 [47–87]* | 78/22 | 14/44 | 100 | 81 | 100 | LHR: 23 [10–30]*, TA: 21 [21–30]* |
| Yamashita | LHR: 61±9, TA: 66±9 | 65/35 | 14/75 | 100 | 87 | 74 | LHR: 24±9, TA: 20±6 |
*, median [range]; °, median [interquartile range]; §, Child-Pugh score (points: mean ± SD). TA, thermo-ablation; LHR, laparoscopic hepatic resection.
Complications
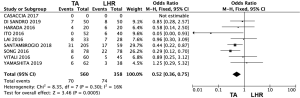
The postoperative complications were evaluated in 8 studies (Figure 1) (17-21,23-25). The meta-analysis results showed that the TA group had a significantly lower morbidity rate in comparison with that of the LHR group (OR: 0.52, 95% CI: 0.36–0.75, P=0.0005). Only 6 studies classified complications according Dindo-Clavien classification: two studies (24,25) considered a score >2 while the other four studies (17,18,20,23) a score >3. In these studies, severe complications occurred in a range of 0–10% in the LHR group and in a range of 0–5% in the TA group. No postoperative mortality has been shown in both groups.
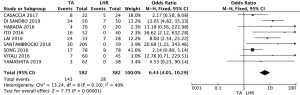
Local recurrences
Local recurrence was defined as intrahepatic recurrence, including the presence of viable tumor contiguous of resection area or of the ablated HCC nodule. The meta-analysis of these studies showed that the LHR group had lower local recurrence rates than the TA group (OR: 6.43; 95% CI: 4.01–10.29; P<0.00001) (Figure 2).
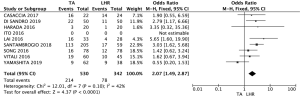
OS
Eight of the nine studies selected reported mortality data (Figure 3) (17,19-25). Meta-analysis results showed that the patients who underwent LHR had significantly better OS rates than the patients who submitted to TA (OR: 2.07; 95% CI: 1.49–2.87; P<0.00001). Only the study by Yamashita et al. reported a slight better 5-year OS rate for TA than for LHR.
Discussion
Although an increasing number of randomized trials can be found in the surgical Literature, non-randomized studies, both comparative and noncompetitive studies are usually performed in surgery. In consideration of the absence of RCTs comparing TA and LHR, a meta-analytical analysis using non-RCTs might be a valid method to furnish a scientific opinion in this field.
Recent meta-analyses showed that HR offers better long-term oncologic outcomes in comparison with TA, also in patients with small HCC nodules (<20 mm) which usually indicated for TA (6,7). However, TA patients had fewer postoperative complications and shorter hospitalization duration (8,26). On the other hand, recent studies demonstrated that LHR for HCC seems to be superior to open HR: during the hospital stay, patients submitted to LHR required less intraoperative blood loss, less blood transfusions, and fewer days of hospital stay, while during the long-term follow-up, patients had similar rates of OS, disease-free survival, and recurrence (27). For these reasons, we tried to evaluate if these advantages of LHR in comparison to open HR could influence the postoperative outcome in order to reduce the complications rates after surgery in comparison to a less invasive treatment strategy as TA.
This meta-analysis showed that LHR was superior to TA in terms of OS rate. On the other hand, during the mean follow-up period, the TA group had a higher local recurrence rate than LHR group. However, we demonstrated that the TA group suffered less postoperative complications in comparison of the LHR group, even if severe complications (Dindo-Clavien score >3) do not show a higher rate (28). Furthermore, in these studies no postoperative mortality occurred in both groups.
Actually, the current EASL/AASLD guidelines are very controversial with regard to the selection of HR and TA as the first-line therapy for small HCC due to absence of well-designed randomized trials (1), which the reasons may be related to poor patient enrollment and difficulty in randomization, as well as ethical concerns. Analysis of comparative studies demonstrates superiority of HR in overall and disease-free survival (29). However, TA is less invasive, has lower complication rates (30-33), a lower cost (34), and results in shorter hospital stays (15,32,33), which may make it a preferable alternative to HR. On the other hand, many previous studies demonstrated the feasibility and safety of LHR (13,14,27). Recently, the Southampton Guidelines (35) have established that when performed by expert surgeons, LHR offers significant advantages in patients with cirrhosis reducing the risk of postoperative ascites and liver failure. They also advocate that the LHR should be considered the gold standard for tumors in the left lateral and the anterior segments. In this setting, LHR could be a valid alternative indication to TA for patients with small HCC nodules. In fact, the association of mini-invasive approach and radical oncologic treatment could guarantee for LHR better long-term outcomes than TA (36,37). In fact, IUOS examination permits to identify satellite lesions adjacent to primary nodule and venous tumor thrombi: therefore, their eradications with LHR, promoting a better long-term survival compared with TA (38). In the same setting, the laparoscopic IOUS during a TA procedure permits to identify these HCC nodules with microinvasive behavior (39): in these cases, a microwave ablation, instead of a radiofrequency ablation, could produce a wider ablation area including vascular micro-infiltration and satellite micronodules and decreasing probability of metastasis of the residual tumor cell by intrahepatic portal vein (39). Furthermore, by adopting laparoscopic and open approaches, tumor lesions are easier to find, which is the weak point of percutaneous TA, which could probably miss HCC nodules when there are more than one lesion (40). The survived nodules might be the cause of HCC recurrence and lower survival rates.
In conclusion, our metanalysis reveals that LHR group got significantly higher OS rates and lower local recurrence rates than TA. However, even if several studies showed that LHR is a safer treatment than open HR, TA treatments have a low impact in the postoperative course of cirrhotic patients. For these reasons TA could be an effective approach to treat HCC, but not yet good enough to replace LHR, as better OS rates are observed in patients underwent LHR. In the future guidelines, LHR should have a primary role in first-line treatment for HCC, while in the situations that the condition of patients is not suitable to perform operation, TA might be the solution. Laparoscopic TA or different surgical approaches for the ablation of HCC nodules from problematic locations should be considered in larger future studies.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2019.11.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Sala M, Fuster J, Llovet JM, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl 2004;10:1294-300. [Crossref] [PubMed]
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132-40. [Crossref] [PubMed]
- Ferrer-Fàbrega J, Forner A, Liccioni A, et al. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology 2016;63:839-49. [Crossref] [PubMed]
- Feng Q, Chi Y, Liu Y, et al. Efficacy and safety of percutaneous radiofrequency ablation versus surgical resection for small hepatocellular carcinoma: a meta-analysis of 23 studies. J Cancer Res Clin Oncol 2015;141:1-9. [Crossref] [PubMed]
- Ferrer-Fàbrega J, Chan JA, Aloia TA, et al. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer 2017;123:1817-27. [Crossref] [PubMed]
- Yin Z, Jin H, Ma T, et al. A meta-analysis of long-term survival outcomes between surgical resection and radiofrequency ablation in patients with single hepatocellular carcinoma ≤ 2 cm (BCLC very early stage). Int J Surg 2018;56:61-7. [Crossref] [PubMed]
- Uhlig J, Sellers CM, Stein SM, et al. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National Cancer Database. Eur Radiol 2019;29:2679-89. [Crossref] [PubMed]
- Kim PN, Choi D, Rhim H, et al. Planning ultrasound for percutaneous radiofrequency ablation to treat (<3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: a multicenter prospective study to assess factors affecting ultrasound visibility. J Vasc Interv Radiol 2012;23:627-34. [Crossref] [PubMed]
- Kim JE, Kim YS, Rhim H, et al. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol 2011;79:e80-4. [Crossref] [PubMed]
- Rhim H, Lee MH, Kim YS, et al. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR 2008;190:1324-30. [Crossref] [PubMed]
- Aldrighetti L, Belli G, Boni L, et al. Italian experience in minimally invasive liver surgery: a national survey. Updates Surg 2015;67:129-40. [Crossref] [PubMed]
- Tanaka S, Takemura S, Shinkawa H, et al. Outcomes of pure laparoscopic versus open hepatic resection for hepatocellular carcinoma in cirrhotic patients: a case-control study with propensity score matching. Eur Surg Res 2015;55:291-301. [Crossref] [PubMed]
- Zhou YM, Shao Y, Zhao YF, et al. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci 2011;56:1937-43. [Crossref] [PubMed]
- Santambrogio R, Bruno S, Kluger MD, et al. Laparoscopic ablation therapies or hepatic resection in cirrhotic patients with small hepatocellular carcinoma. Dig Liver Dis 2016;48:189-96. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Harada N, Maeda T, Yoshizumi T, et al. Laparoscopic liver resection is a feasible treatment for patients with hepatocellular carcinoma and portal hypertension. Anticancer Res 2016;36:3489-97. [PubMed]
- Ito T, Tanaka S, Iwai S, et al. Outcomes of laparoscopic hepatic resection versus percutaneous radiofrequency ablation for hepatocellular carcinoma located at the liver surface: a case-control study with propensity score matching. Hepatol Res 2016;46:565-74. [Crossref] [PubMed]
- Lai C, Jin RA, Liang X, et al. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B 2016;17:236-46. [Crossref] [PubMed]
- Vitali GC, Laurent A, Terraz S, et al. Minimally invasive surgery versus percutaneous radio frequency ablation for the treatment of single small (</=3 cm) hepatocellular carcinoma: a case-control study. Surg Endosc 2016;30:2301-7. [Crossref] [PubMed]
- Song J, Wang Y, Ma K, et al. Laparoscopic hepatectomy versus radiofrequency ablation for minimally invasive treatment of single, small hepatocellular carcinomas. Surg Endosc 2016;30:4249-57. [Crossref] [PubMed]
- Casaccia M, Santori G, Bottino G, et al. Laparoscopic resection vs laparoscopic radiofrequency ablation for the treatment of small hepatocellular carcinomas: A single-center analysis. World J Gastroenterol 2017;23:653-60. [Crossref] [PubMed]
- Santambrogio R, Barabino M, Bruno S, et al. Surgical resection versus ablative therapies through a laparoscopic approach for hepatocellular carcinoma: a comparative study. J Gastrointest Surg 2018;22:650-60. [Crossref] [PubMed]
- Di Sandro S, Benuzzi L, Lauterio A, et al. Single Hepatocellular Carcinoma approached by curative-intent treatment: A propensity score analysis comparing radiofrequency ablation and liver resection. Eur J Surg Oncol 2019;45:1691-9. [Crossref] [PubMed]
- Yamashita YI, Imai K, Kaida T, et al. Multimodal radiofrequency ablation versus laparoscopic hepatic resection for the treatment of primary hepatocellular carcinoma within Milan criteria in severely cirrhotic patients: long-term favorable outcomes over 10 years. Surg Endosc 2019;33:46-51. [Crossref] [PubMed]
- Xu XL, Liu XD, Liang M, et al. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology 2018;287:461-72. [Crossref] [PubMed]
- Jiang B, Yan XF, Zhang JH. Meta-analysis of laparoscopic versus open liver resection for hepatocellular carcinoma. Hepatol Res 2018;48:635-63. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 633 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Jia JB, Zhang D, Ludwig JM, et al. Radiofrequency ablation versus resection for hepatocellular carcinoma in patients with Child-Pugh A liver cirrhosis: a meta-analysis. Clin Radiol 2017;72:1066-75. [Crossref] [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [Crossref] [PubMed]
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. [Crossref] [PubMed]
- Peng ZW, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology 2012;262:1022-33. [Crossref] [PubMed]
- Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89-97. [Crossref] [PubMed]
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300-7. [Crossref] [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Li X, Wu YS, Chen D, et al. Laparoscopic hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Manag Res 2019;11:5711-24. [Crossref] [PubMed]
- Si MB, Yan PJ, Hao XY, et al. Efficacy and safety of radiofrequency ablation versus minimally invasive liver surgery for small hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc 2019;33:2419-29. [Crossref] [PubMed]
- Yamashita Y, Tsuijita E, Takeishi K, et al. Predictors for microinvasion of small hepatocellular carcinoma <2 cm. Ann Surg Oncol 2012;19:2027-34. [Crossref] [PubMed]
- Santambrogio R, Cigala C, Barabino M, et al. Intraoperative ultrasound for prediction of hepatocellular carcinoma biological behaviour: Prospective comparison with pathology. Liver Int 2018;38:312-20. [Crossref] [PubMed]
- Santambrogio R, Barabino M, Bruno S, et al. Long-term outcome of laparoscopic ablation therapies for unresectable hepatocellular carcinoma: a single European center experience of 426 patients. Surg Endosc 2016;30:2103-13. [Crossref] [PubMed]
Cite this article as: Santambrogio R, Barabino M, D’Alessandro V, Galfrascoli E, Zappa MA. Does laparoscopic liver resection provide better outcomes than thermoablation in patients with hepatocellular carcinoma?—A systematic review. Laparosc Surg 2020;4:9.


