
Minimally invasive approaches to hepatic arterial infusion pump placement in metastatic colorectal cancer
Introduction
Colorectal cancer is the third most common malignancy and the third most common cause of cancer-related death in the United States. Most patients die from distant metastases, and 60% will develop liver metastases over the course of their disease (1,2). While curative-intent hepatectomy offers the best chance for long-term survival, fewer than 25% of patients are resectable (3). Over the last four decades, multimodal medical and surgical treatments have been developed in an effort to address historically modest response rates to systemic chemotherapy. It is in this setting that hepatic arterial infusion pump (HAIP) chemotherapy has emerged as an effective and unique form of regional chemotherapy.
Over the past five decades, the safety and efficacy of HAIP chemotherapy has been evaluated. Randomized control led trials conducted in the setting of unresectable liver metastasis have demonstrated improved response rates, quality of life, and overall survival (4-10). Studies examining the use of HAIP chemotherapy in the adjuvant setting have also shown benefit, with improved hepatic recurrence rates and survival (11,12).
Traditionally, HAIP placement has been performed via an open approach (13). In the context of unresectable metastatic disease, the morbidity of a laparotomy has been a limiting aspect of HAIP chemotherapy (14). The rising prevalence of laparoscopic and robotic approaches to colectomy and hepatectomy in colorectal cancer surgery represent yet another impulse to adopt minimally invasive (MIS) approaches to HAIP placement.
Herein, we describe the development of surgical approaches to HAIP placement, including the emergence of MIS HAIP surgery, relevant pre- and post-operative considerations, salient operative details in the MIS approach, and common complications related to HAIP placement and chemotherapy.
Historical development of hepatic arterial infusion chemotherapy
Anatomical and pathological research beginning in the 1950s established the dual blood supply of liver and the preferential arterial blood supply of intrahepatic tumors (15). Normal hepatocytes derive nutrients from portal venous blood flow, while liver tumors obtain nutrients almost exclusively from the hepatic artery.
In the 1960s, Sullivan and colleagues at the Lahey clinic made use of these insights to pioneer surgical techniques for inserting hepatic arterial catheters for regional delivery of chemotherapy. Though a small cohort with both heterogeneous tumor types and chemotherapeutic agents, they reported two important findings: (I) tumors showed favorable treatment response to regional chemotherapy, and (II) 5-fluoro-2’deoxyuridine (FUDR)—with its high first pass liver extraction and short half-life—maximized local efficacy while limiting systemic toxicity. Their work established the feasibility and general principles of surgical access for HAIP chemotherapy (16).
Initial HAIP chemotherapy protocols utilized an external pump system. This required either long-term hospitalization for continuous infusion, or the use of a bulky and cumbersome pump/harness with self-administration of chemotherapeutic drugs by patients (17). The development of a fully implantable pump system by Ensminger and colleagues by the 1980s transformed hepatic arterial chemotherapy into a truly outpatient treatment modality (18) (Figure 1).
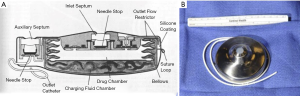
Subsequent publications highlighted that although potentially beneficial, hepatic arterial chemotherapy was not without risk. In a period before stringent patient selection criteria, and with variable use of fully implantable pump systems, and procedural variation, mortality and major morbidity from surgery varied widely, with rates ranging from ~0–17% and 12–41%, respectively (20-25).
In the following decades, several developments led to improved safety and outcomes for HAIP surgery. These included formal criteria for patient selection, refinement in the management of variant hepatic arterial anatomy, and improved salvage of HAIP chemotherapy after complications (19,26-31). In modern series with good patient selection and confirmed in subsequent meta-analyses, mortality rates have approached 0%, with overall and pump-related complication rates reported between 10–20% (26,29).
One of the concerns regarding HAIP chemotherapy has been the morbidity associated with laparotomy in patients already debilitated with cancer, especially in the unresectable metastatic setting (32,33). With the emergence of laparoscopic surgery, several small series were published in the 1990s reporting the safety and feasibility of laparoscopic HAIP insertion as an alternative to laparotomy (34,35). To date, four series examining laparoscopic HAIP surgery and one examining robotic HAIP placement have been published (14,36-39). A single study has examined comparative outcomes in open vs. MIS approaches (39).
Overall rates of mortality for MIS HAIP surgeries are comparable to the open approach and range from 0–3%. As with open approaches, this rate is difficult to interpret given the frequency of concomitant colorectal and liver surgeries. Overall complication rates are likewise similar, ranging from 12–21%. Operative duration, estimated blood loss (EBL), and complication rates are similar to open HAIP placement (Table 1).
Table 1
| Author | Year | Study type | Approach | N | EBL (cc) | Concurrent surgical procedure | Convert to open | Median LOS (days) | Peri-op death | Reported complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Qadan |
2017 | RCS | Open | 53 | 369 | Yes | NA | 6 | 0 | 40: 27, grades 1–2; 13, grades 3–5 |
| 200 | HAIP alone | |||||||||
| Lap | 21 | 160 | Yes | 14 | 5 | 0 | 5: grades 1–2 | |||
| 168 | HAIP alone | |||||||||
| Robot | 24 | 170 | Yes | 4 | 4 | 0 | 19: 15, grades 1–2; 4, grades 3–5 | |||
| 81 | HAIP alone | |||||||||
| Dhir |
2016 | RCS | Robot | 24 | 100 (IQR 20–200) | Yes | 1 | NR | 8: 5, grades 1–2; 3, grades 3–5 | |
| Franklin |
2006 | RCS | Lap | 27 | 151 (range 20–300) | Yes | NR | 8.4 | 1, multi-system organ failure; 1, bleeding; 3, catheter related; 1, catheter thrombosis; 1, partial catheter occlusion; 1, duodenal erosion | |
| Cheng |
2004 | RCS | Lap | 38 | 100 (range 25–1,200) | Yes | 1 | 3 | 2, ileus; 5, catheter related; 3, catheter thrombosis; 1, pulmonary embolus; 1, pump replacement | |
| Urbach |
2001 | RCS | Lap | 8 | 50 | Yes | 1 | 4 | 0 | 1, pulmonary embolus |
| Allen |
2005 | RCS | Open | 544 | 314 (SD 303) | yes | NA | 5 | 120, non-catheter related; 49, catheter related; 33, arterial thrombosis; 16, extrahepatic perfusion |
HAIP, hepatic arterial infusion pump; RCS, retrospective case series; EBL, estimated blood loss; Lap, laparoscopic; LOS, length of stay.
Pre-operative evaluation
Pre-operative evaluation of all potential HAIP chemotherapy patients—regardless of operative approach—involves staging of disease; multidisciplinary discussion regarding the relative roles of systemic, regional, and local treatment modalities; and formal assessment of medical co-morbidities and surgical risk.
Clinical, endoscopic, and radiographic assessment [multidetector computed tomography (CT) scans without PET or MRI of the abdomen] are typically undertaken to assess the patient’s burden of disease and to rule out extrahepatic disease (EHD) (40). Provided they can adhere to scheduled refilling and necessary follow-up, individuals with good performance status, no obvious sites of EHD, and preserved liver function can be considered for HAIP chemotherapy.
Relative contraindications for placement of HAIP can be broadly classified into: (I) patient, (II) tumor, and (III) anatomic factors (Table 2).
Table 2
| Patient | Tumor | Anatomic factors |
|---|---|---|
| Poor performance status (Karnofsky <60%) | Extrahepatic disease | Portal vein thrombosis |
| Liver insufficiency (total bilirubin >1.5 mg/dL) | Extensive hepatic metastasis >50% | |
| Irreversible coagulopathy | ||
| Current infection | ||
| Inability to follow up for routine follow-up |
HAIP, hepatic arterial infusion pump.
In patients deemed appropriate candidates, pre-operative evaluation of the patient’s hepatic arterial anatomy is paramount. One of the main aims of pre-operative assessment of hepatic arterial anatomy is to identify potential variant arterial anatomy. Such findings necessarily shape the operative plan and may alter the point of arterial cannulation for insertion of the HAIP. At present, CT angiography represents the gold standard for evaluation of hepatic arterial anatomy, providing high-resolution arterial anatomy without artifacts that is technically adequate for pre-operative planning.
The approach to variant hepatic arterial anatomy
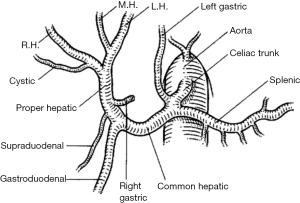
Variant hepatic arterial anatomy has been a well-known phenomenon since the 1960s with the publication of Nicholas Michels’s systematic study of variant anatomy based on 200 cadaveric subjects. Normal hepatic arterial anatomy is defined by: (I) common hepatic arterial origin off the celiac axis, and (II) when the gastroduodenal artery arises from the common hepatic artery (CHA) proximal to the bifurcation of left and right hepatic arteries (Figure 2). This is present only 50–60% of the time. The most common variants involve the presence of “replaced” or “accessory” right (15%) or left (11%) hepatic arterial anatomy (42).
Early approaches to variant hepatic arterial vasculature in HAIP surgery were highly variable. Techniques ranged from ligation of accessory vessels, technically complex anastomoses between gastroduodenal artery (GDA) and replaced or accessory vessels, or retrograde catheterization via the splenic artery (20,30,43). The use of dual lumen pump systems was also utilized; however, extended operative time and uneven hepatic perfusion led to its disuse (44).
The surgical management of variant anatomy was greatly simplified with the concept of ligation of all accessory and replaced vessels. Rayner and colleagues demonstrated the safety of such an approach in 15 patients, with complete bi-lobar hepatic perfusion documented in 87% of patients (27). While early experience with a simplified approach demonstrated higher rates of technical complications in patients with variant anatomy (23), modern series have shown that outcomes are equivalent (44).
In the largest review of variant anatomy, Allen and colleagues examined 265 consecutive HAIP placements over a five-year period to determine whether patients with variant anatomy experienced increased rates of catheter-related complications and to determine optimal technique. They reported an overall morbidity rate of 20% and a 12% rate of pump-related complications. Patients with variant anatomy were more likely to experience pump-related complications if a vessel other than the GDA was cannulated (28% vs. 4%, P<0.001) and if they had multiple variant vessels versus only a single variant vessel (23% vs. 6%, P<0.05) (30). A follow-up study examining technical complications in 544 patients undergoing HAIP insertion reported similar overall mortality and technical complication rates. Of note, on multivariable analysis, only non-GDA insertion of the catheter and surgeon experience (<25 cases) were associated with complications (26).
These results helped standardize the management of variant hepatic arterial anatomy by establishing the safety and efficacy of ligating all variant vessels and underscored the importance of GDA insertion of HAIP catheters whenever possible. The techniques standardized in open surgery serve as the foundation for MIS HAIP surgery.
The procedure
Once a patient is determined to potentially benefit from HAIP chemotherapy, is found to be a reasonable surgical candidate, and found to have suitable hepatic arterial anatomy on pre-operative imaging, the patient is taken to the operating room.
The patient is placed in supine position with a footboard, general anesthesia is induced, and the patient is prepped and draped in sterile fashion. Selection of the pump site is performed early in the operation and marked with a 7–8 cm transverse line—typically on the left side—2–3 finger breadths below the costal margin and above the anterior superior iliac spine. When patients are morbidly obese, the pocket is created above the costal margin allowing the pump to rest on the left chest wall to minimize pump migration and flipping. Marking of the pump pocket at this stage of the operation avoids inadvertent placement of trocars through the eventual pump pocket (Figure 3).
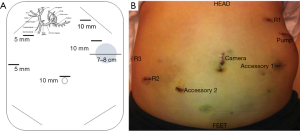
After obtaining intraperitoneal access and performing diagnostic laparoscopy to rule out EHD, the patient is placed into steep reverse Trendelenburg. Cholecystectomy is then performed to prevent chemotherapy induced cholecystitis. In cases where hepatic arterial anatomy is normal (the most common scenario), the common hepatic (CHA), GDA, proper hepatic artery (PHA), right hepatic artery (RHA), and left hepatic artery (LHA) are identified. When hepatic arterial anatomy is variant, the accessory or replaced artery is ligated (Figure 4). Sharp dissection with hook cautery is initiated 2 cm proximal to the takeoff of the GDA. The distal CHA, GDA, and proper hepatic artery (PHA) are circumferentially mobilized, and the right gastric artery is ligated. All accessory and collateral branches should be ligated to prevent extrahepatic perfusion. The LHA should be cleared up to first order branches, and the RHA dissected and cleared as far as is safe and feasible, as systematic review of cases of extrahepatic perfusion revealed that collaterals off the RHA account for the overwhelming majority of cases (45).
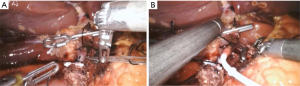
In nearly all instances, the pump catheter can be inserted in the GDA, which is the preferred vessel for pump catheter insertion. Doing so maximizes bi-lobar perfusion, minimizes turbulent arterial flow, and has been associated with long-term patency of the pump (30).
Pneumoperitoneum is released and a subcutaneous pump pocket is created at the site marked earlier in the case. Subcutaneous flaps are raised as needed to accommodate the pump, and the pocket should remain superficial to the fascia to facilitate needle access at subsequent oncology visits. Only after the GDA is ready for cannulation and the pump pocket created is the pump device brought into the surgical field. It is first filled with heparinized saline and the catheter copiously flushed with heparinized saline. A small rent in the fascia is made in the center of the pocket and the catheter is fed into the abdominal cavity. The pump is placed atop the remaining extraperitoneal length of catheter and, after ensuring the catheter has a straight unobstructed path into the peritoneal cavity, the pump is secured to the fascia with stay sutures.
Pneumoperitoneum is re-established and attention is then turned to cannulating and securing the catheter into the GDA. The CHA, GDA, and PHA are coated with papaverine injected through the abdominal wall with a spinal needle. Control of the distal GDA is obtained by ligation with non-absorbable suture and a surgical clip. Proximal control is achieved with small, straight bulldog clamps inserted through a 12 mm port, which are then placed on the CHA and PHA. Alternatively, a single curved bulldog clamp can be placed on the CHA/PHA to exclude flow to the GDA and allow for placement of the catheter with the tip at the junction of the GDA with the CHA/PHA. A non-absorbable suture is placed posterior to the GDA and will be used to secure the catheter once introduced.
An arteriotomy is made with a No. 11 blade in the anterior wall of the GDA and a plastic introducer is used to facilitate introduction of the catheter into the GDA to avoid dissection. The previously placed suture is tied to secure the catheter in place. An additional two ties are then placed around the GDA to avoid catheter migration (Figure 4).
Once the catheter is in place, adequacy of hepatic perfusion is assessed by injecting methylene blue into the pump. The liver is grossly assessed for uniform color change immediately post injection and after several minutes, making note of the presence of any extrahepatic perfusion.
After ensuring the absence of kinking in the catheter and confirming easy flushing of the pump with heparinized saline, ports are removed under direct visualization and pneumoperitoneum is released. All port sites larger than 8 mm are closed at the level of the fascia. Finally, all port sites closed with absorbable suture.
Confirming adequacy of hepatic perfusion
Prior to the first dose of HAIP chemotherapy, appropriate hepatic perfusion via pump is evaluated by means of a radionuclide pump-flow study. Radiolabeled sulfur-colloid (SC) is injected intravenously and technetium-labeled macroaggregated albumin (MAA) via the pump. Perfusion scans are obtained and the two images overlaid: SC representing intrahepatic perfusion and MAA the region perfused by the pump. If the fused study is normal, HAI chemotherapy is initiated, usually 1–4 weeks post-operatively (46).
Incomplete hepatic perfusion occurs in 2% of cases and typically results from failure to ligate an aberrant vessel or failure of cross lobar collateralization. These patients are followed with repeat perfusion scans in 2–4 weeks, and nearly all have resolution of incomplete perfusion (30). In instances where this is not the case, angiography with embolization of any remaining aberrant vessels can be performed.
Extrahepatic perfusion may be detected on the post-operative perfusion scan or based on clinical presentation during chemotherapy. The MAA-labeled scan will demonstrate an area of perfusion beyond the territory delineated in the SC scan, indicating the potential for perfusion of the duodenum, pancreas, or stomach. In cases not detected prior to initiation of HAIP chemotherapy, severe epigastric pain or diarrhea with infusion is typical. The underlying etiology of such symptoms is pancreatitis or ulcers. Up to 80% of pumps with extrahepatic perfusion can be salvaged with endovascular embolization procedures (47).
Post-operative/technical complications
Large single-institution case series as well as several meta-analyses have been published characterizing the complication profile of HAIP surgery in detail. Pump-related complication rates in these modern analyses range from 10–20%. The most common complications are arterial thrombosis (6–7%), perfusion abnormalities (5%), catheter dislodgment (3–5%), and pump pocket infection (1–3%) (26,29,48,49). In the most comprehensive series evaluating technical complications in 544 consecutive HAIP placements (open and MIS approaches), the overall salvage rate for complications was 45% and the complication-related HAIP failure rate was 12% (26).
Complications from MIS HAIP insertion are similar to the open technique (Table 1). Where published, rates of catheter-related complications in laparoscopic HAIP range from 11–13% (14,37,38). Overall, the majority of complications are mild, with 60-100% of complications reported as grade 1–2, with few grade 3–5 complications reported (36,38,39). To date, there have been no reported differences in rates of complications.
Of relevance in evaluating MIS approaches to HAIP insertion is the conversion rate to open. Rates of conversion range from 3–67% (14,36-39) in laparoscopic cases. Conversion during robotic operations is similar, with reported rates of 4–17% (38,39). Of note, in the only study systematically comparing open, laparoscopic, and robotic approaches to HAIP insertion, conversion rates of robotic approaches were significantly lower than laparoscopic (17% vs. 67%, P<0.01), with no differences in overall complication rate or length of stay.
Chemotherapy/drug-related toxicity
The adverse event profile of HAIP chemotherapy is unique in that patients experience both technical- and chemotherapy-related toxicities. FUDR and 5FU are the most commonly used regional chemotherapeutic agents in HAIP chemotherapy. With almost entire first pass liver extraction, systemic toxicity from FUDR is limited and adverse events are local. Chemical hepatitis is the most common adverse event in FUDR-based regimens (34%), and typically presents as liver function test abnormalities (29).
By contrast, 5FU extraction by the liver is modest, ranging from 20–40%, and common adverse events relate to systemic toxicities. The most commonly reported adverse events are gastrointestinal, with nausea, vomiting, and diarrhea reported in 40% of patients (29). For these patients, the dosing schedule is modified and treatment resumes with normalization of lab work, as outlined in published dosing guidelines (50).
Biliary toxicity is a related but distinct complication of HAIP chemotherapy and occurs in 5–30% of patients (29,51). Unlike the portal venous-based perfusion of hepatocytes, the biliary tree depends on arterial perfusion. HAIP chemotherapy, therefore, places patients at significant potential risk for biliary toxicity and injury. The use of dexamethasone with FUDR has been shown to mitigate the potential biliary toxicity of FUDR HAIP chemotherapy and is a standard part of current FUDR-based regimens (52).
The most significant and serious manifestation of biliary toxicity is biliary sclerosis. Published incident rates of biliary sclerosis in the setting of HAIP chemotherapy range from 1–26% and are more prevalent in the adjuvant setting, with FUDR, and with co-administration of the anti-VEGF drug bevacizumab (53). Based on published results, the etiology is thought to be related to ischemic and drug toxicity.
Early recognition of biliary toxicity and prompt intervention in cases of biliary sclerosis are critical in preventing long-term harm. Should dose modification or cessation (temporary or definitive) fail to resolve laboratory abnormalities, radiographic studies (CT or MRI) should be obtained to discover potential strictures, which may be treated with endoscopic retrograde cholangiopancreatographic stent/dilation. In patients with isolated strictures from biliary sclerosis who can be stented or dilated, survival is not compromised (51).
Summary
Since its initial development, HAIP chemotherapy has emerged as an important therapeutic modality in the treatment of colorectal cancer liver metastasis. With standardized patient selection, operative technique, and familiarity with post-operative pump-related complications, published outcomes have improved and are more consistent. Laparoscopic HAIP surgery has comparable safety and efficacy to standard open approaches.
The role of laparoscopic HAIP in an era of robotic surgery remains an outstanding question. Two published series report comparable outcomes for robotic HAIP placement (38,39). Rates of conversion, however, do appear to be lower in robotic approaches. It does appear to be a procedure that is ideal for the robotic platform, given the small operative field that requires very fine vascular dissection. As an operative platform, the articulating, wristed movement of robotic instruments is particularly well-suited for cannulating and securing the arterial catheter. At our own institution, we no longer routinely perform laparoscopic HAIP placement, favoring the robotic approach (Figure 4). On the other hand, laparoscopic HAIP surgery remains an important MIS surgical technique in the setting of concomitant laparoscopic colorectal or liver surgery. Regardless of approach, surgeon and institutional experience with HAIP chemotherapy remain critical factors for ensuring optimal outcomes and rescuing patients from pump-related complications.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (H. Leon Pachter) for the series “Laparoscopic Surgery of the Liver and Spleen” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2019.11.07). The series “Laparoscopic Surgery of the Liver and Spleen” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin 1999;49:8-31, 1.
- Stratmann SL. Hepatic artery chemotherapy in the management of colorectal metastases. Proc (Bayl Univ Med Cent) 2002;15:376-9. [Crossref] [PubMed]
- Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24:1395-403. [Crossref] [PubMed]
- Allen-Mersh TG, Earlam S, Fordy C, et al. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet 1994;344:1255-60. [Crossref] [PubMed]
- Rougier P, Laplanche A, Huguier M, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol 1992;10:1112-8. [Crossref] [PubMed]
- Martin JK Jr, O'Connell MJ, Wieand HS, et al. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg 1990;125:1022-7. [Crossref] [PubMed]
- Wagman LD, Kemeny MM, Leong L, et al. A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol 1990;8:1885-93. [Crossref] [PubMed]
- Hohn DC, Stagg RJ, Friedman MA, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol 1989;7:1646-54. [Crossref] [PubMed]
- Kemeny N, Daly J, Reichman B, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med 1987;107:459-65. [Crossref] [PubMed]
- Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999;341:2039-48. [Crossref] [PubMed]
- Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol 2002;20:1499-505. [PubMed]
- Kemeny N, Fata F. Hepatic-arterial chemotherapy. Lancet Oncol 2001;2:418-28. [Crossref] [PubMed]
- Cheng J, Hong D, Zhu G, et al. Laparoscopic placement of hepatic artery infusion pumps: technical considerations and early results. Ann Surg Oncol 2004;11:589-97. [Crossref] [PubMed]
- Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol 1954;30:969-77. [PubMed]
- Sullivan RD, Zurek WZ. Chemotherapy for liver cancer by protracted ambulatory infusion. Jama 1965;194:481-6. [Crossref] [PubMed]
- Dorr RT, Trinca CE, Griffith K, et al. Limitations of a portable infusion pump in ambulatory patients receiving continuous infusions of anticancer drugs. Cancer Treat Rep 1979;63:211-3. [PubMed]
- Ensminger W, Niederhuber J, Dakhil S, et al. Totally implanted drug delivery system for hepatic arterial chemotherapy. Cancer Treat Rep 1981;65:393-400. [PubMed]
- Leal JN, Kingham TP. Hepatic artery infusion chemotherapy for liver malignancy. Surg Oncol Clin N Am 2015;24:121-48. [Crossref] [PubMed]
- Cady B. Hepatic arterial patency and complications after catheterization for infusion chemotherapy. Ann Surg 1973;178:156-61. [Crossref] [PubMed]
- Daly JM, Kemeny N, Oderman P, et al. Long-term hepatic arterial infusion chemotherapy. Anatomic considerations, operative technique, and treatment morbidity. Arch Surg 1984;119:936-41. [Crossref] [PubMed]
- Heinrich S, Petrowsky H, Schwinnen I, et al. Technical complications of continuous intra-arterial chemotherapy with 5-fluorodeoxyuridine and 5-fluorouracil for colorectal liver metastases. Surgery 2003;133:40-8. [Crossref] [PubMed]
- Campbell KA, Burns RC, Sitzmann JV, et al. Regional chemotherapy devices: effect of experience and anatomy on complications. J Clin Oncol 1993;11:822-6. [Crossref] [PubMed]
- Barone RM, Byfield JE, Goldfarb PB, et al. Intra-arterial chemotherapy using an implantable infusion pump and liver irradiation for the treatment of hepatic metastases. Cancer 1982;50:850-62. [Crossref] [PubMed]
- Lucas RJ, Tumacder O, Wilson GS. Hepatic artery occlusion following hepatic artery catheterization. Ann Surg 1971;173:238-43. [Crossref] [PubMed]
- Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg 2005;201:57-65. [Crossref] [PubMed]
- Rayner AA, Kerlan RK, Stagg RJ, et al. Total hepatic arterial perfusion after occlusion of variant lobar vessels: implications for hepatic arterial chemotherapy. Surgery 1986;99:708-15. [PubMed]
- Skitzki JJ, Chang AE. Hepatic artery chemotherapy for colorectal liver metastases: technical considerations and review of clinical trials. Surg Oncol 2002;11:123-35. [Crossref] [PubMed]
- Barnett KT, Malafa MP. Complications of hepatic artery infusion: a review of 4580 reported cases. Int J Gastrointest Cancer 2001;30:147-60. [Crossref] [PubMed]
- Allen PJ, Stojadinovic A, Ben-Porat L, et al. The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol 2002;9:875-80. [Crossref] [PubMed]
- Curley SA, Chase JL, Roh MS, et al. Technical considerations and complications associated with the placement of 180 implantable hepatic arterial infusion devices. Surgery 1993;114:928-35. [PubMed]
- Kemeny N, Daly J, Oderman P, et al. Hepatic artery pump infusion: toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol 1984;2:595-600. [Crossref] [PubMed]
- Curley SA, Hohn DC, Roh MS. Hepatic artery infusion pumps: cannulation techniques and other surgical considerations. Langenbecks Arch Chir 1990;375:119-24. [Crossref] [PubMed]
- Franklin J. Laparoscopic approach for regional hepatic chemotherapy in the treatment of primary or metastatic malignancy. In: Green F. editor. Minimal Access Surgical Oncology. 1 ed. New York: Radcliffe Medical Press, 1995:153-7.
- Feliciotti F, Paganini A, Guerrieri M, et al. Laparoscopic intra-arterial catheter implantation for regional chemotherapy of liver metastasis. Surg Endosc 1996;10:449-52. [Crossref] [PubMed]
- Urbach DR, Hansen PD. Laparoscopic placement of a continuous hepatic artery infusion pump. Semin Laparosc Surg 2000;7:140-7. [Crossref] [PubMed]
- Franklin M, Trevino J, Hernandez-Oaknin H, et al. Laparoscopic hepatic artery catheterization for regional chemotherapy: is this the best current option for liver metastatic disease? Surg Endosc 2006;20:554-8. [Crossref] [PubMed]
- Dhir M, Zenati MS, Padussis JC, et al. Robotic assisted placement of hepatic artery infusion pump is a safe and feasible approach. J Surg Oncol 2016;114:342-7. [Crossref] [PubMed]
- Qadan M, D'Angelica MI, Kemeny NE, et al. Robotic hepatic arterial infusion pump placement. HPB (Oxford) 2017;19:429-35. [Crossref] [PubMed]
- Moulton CA, Gu CS, Law CH, et al. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. Jama 2014;311:1863-9. [Crossref] [PubMed]
- Chamberlain RS. Essential Functional Hepatic and Biliary Anatomy for the Surgeon, Hepatic Surgery, Hesham Abdeldayem, IntechOpen. DOI:
10.5772/53849 . Available online: https://www.intechopen.com/books/hepatic-surgery/essential-functional-hepatic-and-biliary-anatomy-for-the-surgeon. February 13th 2013. - Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337-47. [Crossref] [PubMed]
- Watkins E Jr, Khazei AM, Nahra KS. Surgical basis for arterial infusion chemotherapy of disseminated carcinoma of the liver. Surg Gynecol Obstet 1970;130:581-605. [PubMed]
- Kemeny MM, Hogan JM, Goldberg DA, et al. Continuous hepatic artery infusion with an implantable pump: problems with hepatic artery anomalies. Surgery 1986;99:501-4. [PubMed]
- Perez DR, Kemeny NE, Brown KT, et al. Angiographic identification of extrahepatic perfusion after hepatic arterial pump placement: implications for surgical prevention. HPB (Oxford) 2014;16:744-8. [Crossref] [PubMed]
- Kingham TP, D'Angelica M, Kemeny NE. Role of intra-arterial hepatic chemotherapy in the treatment of colorectal cancer metastases. J Surg Oncol 2010;102:988-95. [Crossref] [PubMed]
- Sofocleous CT, Schubert J, Kemeny N, et al. Arterial embolization for salvage of hepatic artery infusion pumps. J Vasc Interv Radiol 2006;17:801-6. [Crossref] [PubMed]
- Bacchetti S, Pasqual E, Crozzolo E, et al. Intra-arterial hepatic chemotherapy for unresectable colorectal liver metastases: a review of medical devices complications in 3172 patients. Med Devices (Auckl) 2009;2:31-40. [Crossref] [PubMed]
- Scaife CL, Curley SA, Izzo F, et al. Feasibility of adjuvant hepatic arterial infusion of chemotherapy after radiofrequency ablation with or without resection in patients with hepatic metastases from colorectal cancer. Ann Surg Oncol 2003;10:348-54. [Crossref] [PubMed]
- Power DG, Kemeny NE. The role of floxuridine in metastatic liver disease. Mol Cancer Ther 2009;8:1015-25. [Crossref] [PubMed]
- Ito K, Ito H, Kemeny NE, et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: incidence, clinical features, and risk factors. Ann Surg Oncol 2012;19:1609-17. [Crossref] [PubMed]
- Kemeny N, Seiter K, Niedzwiecki D, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer 1992;69:327-34. [Crossref] [PubMed]
- Cercek A, D'Angelica M, Power D, et al. Floxuridine hepatic arterial infusion associated biliary toxicity is increased by concurrent administration of systemic bevacizumab. Ann Surg Oncol 2014;21:479-86. [Crossref] [PubMed]
Cite this article as: Shin PJ, Kingham TP. Minimally invasive approaches to hepatic arterial infusion pump placement in metastatic colorectal cancer. Laparosc Surg 2020;4:5.


