
Glissonian approach for pure laparoscopic donor hepatectomy
Introduction
Laparoscopic liver resection (LLR) is well established as a treatment method for liver disease. This method has been increasingly adopted due to a number of associated benefits, including reduced intraoperative bleeding, lesser pain and subsequently lower analgesic requirement, shorter postoperative hospital stays, and improved cosmetic outcomes (1-3). Moreover, the indications for LLR have been increasing, with major LLR becoming a standard procedure in specialized institutions (4-6). On the other hand, living donor liver transplantation (LDLT) has gained broad acceptance as a therapeutic modality. The most important principle of LDLT is both, to make sure of minimal risk and to provide rapid recovery to the healthy donor. These principles can also be achieved with pure laparoscopic donor hepatectomy (PLDH), which reduces the physical burden on patients who undergo donor hepatectomy. Nevertheless, since this technique is still in development, PLDH remains controversial in terms of its safety and efficacy (7,8). This study aimed to determine whether the Glissonian pedicle approach is an appropriate method for PLDH in terms of safety and efficacy.
Methods
We retrospectively reviewed a database of patients undergone LDLT at our institution. The study was approved by institutional ethics board (No: MH2018-555), and informed consent was taken from all the patients.
Surgical procedure
Right liver graft
Donors were placed in the semi-left lateral decubitus position with the reverse Trendelenburg position. The anesthesiologist maintained a low tidal volume, low airway pressure, and low central venous pressure, all of which contributes to reducing blood loss (9). The operator stood on the patient’s right side, while the operative assistant and laparoscopist stood on the left side of the patient. Five trocars were inserted, and CO2 pneumoperitoneum was maintained at 10 mmHg (Figure 1).
The ligaments around the right lobe was dissected. The Glissonian branch for the caudate lobe (G1c) was divided. The hilar Glissonian pedicle was gently separated from the liver. A pair of forceps was inserted from the left edge of the root of the anterior Glissonian pedicle and put out from the dorsal side of the root of the posterior Glissonian pedicle (Figure 2A,B,C). The right Glissonian pedicle was temporarily clamped, resulting in revealing the demarcation line. The route of the middle hepatic vein was detected by ultrasonography.

The encircled Glissonian pedicle functioned as an important landmark during the liver parenchymal transection. The parenchymal transection was proceeded between the demarcation line, the middle hepatic vein, the right Glissonian pedicle, and the inferior vena cava (Figure 2D). These landmarks avoided disorientation, which is one of the disadvantages of LLR. V5 and V8 were divided, and the caudate lobe was transected. After the liver parenchyma was completely transected, the right hepatic vein was encircled.
The vessels in the right Glissonian pedicle were separated. First, the right hepatic artery was exposed and encircled. Next, the right portal vein was also exposed and encircled. Finally, the right hepatic duct was taped by removing the right hepatic artery and right portal vein from the right Glissonian pedicle.
The right hepatic artery was ligated, and then, the right hepatic duct was clipped and divided. The right hepatic artery was divided, and the right portal vein was ligated and divided. The right hepatic vein was divided using a linear stapler. At last, the right liver graft was procured from a suprapubic 9-cm incision.
Left liver graft
Patients were placed in the supine position with the reverse Trendelenburg position.
Four or five ports were placed, and all other setups were consistent with those of the right liver graft.
The left lateral section was mobilized, and the Arantius duct was divided. The hilar Glissonian pedicle was gently separated from the liver. A pair of forceps was inserted from the right edge of the root of the umbilical portion and put out from the cranial side of the divided Arantius duct (Figure 3A,B,C). The left Glissonian pedicle was temporarily clamped, resulting in revealing the demarcation line.
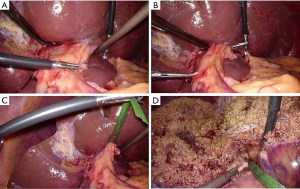
The liver parenchymal transection was performed, during which time the encircled Glissonian pedicle served as an important landmark. The parenchymal transection was proceeded between the demarcation line, the middle hepatic vein, the left Glissonian pedicle, and the Arantius plate (Figure 3D). These landmarks also avoided disorientation. After the parenchymal transection was finished, the left and middle hepatic vein was encircled.
The vessels in the left Glissonian pedicle were separated. First, the left hepatic artery was exposed and encircled. Next, the left portal vein was exposed, and, at the same time, branches of the Spiegel lobe were divided. The left hepatic duct was taped by removing the left hepatic artery and left portal vein from the left Glissonian pedicle.
The left hepatic artery was ligated. Subsequently, the left hepatic duct was ligated and divided. The left hepatic artery was divided, and the left portal vein was ligated and divided. The left and middle hepatic vein was divided using a stapler. At last, the left liver graft was extracted from a suprapubic 7-cm incision.
Results
At our institution, the Glissonian pedicle approach for PLDH was introduced in November 2017, and 14 patients underwent this procedure prior to June 2019. The number of right liver grafts without middle hepatic vein was 10, and the number of left liver grafts with middle hepatic vein was 4. The median surgical time was 384 min (range, 280–563 min), and the median amount of bleeding was 75 mL (range, 21–1,228 mL). The median warm ischemic time was 5 min (range, 2–10 min). Conversion to open laparotomy occurred in one patient (7.1%). A bile leakage of Grade IIIa (according to the Clavien-Dindo classification) occurred in one patient (7.1%).
Discussion
Iwate Medical University is the only center that, to our knowledge, is using a laparoscopic Glissonian pedicle approach for donor hepatectomy. PLDH has not been widely accepted by surgical community due to concerns about both technical difficulty and safety. In other words, PLDH is a technique which is still being developed. Therefore, in order to identify the safest and most effective hepatectomy method, a variety of procedures should be considered.
The Glissonian pedicle approach, which is one method used for anatomical liver resection, was introduced by Couinaud and Takasaki (10,11). This method approaches to the portal pedicle on the hepatic hilum without parenchymal transection. Because various types of anatomical liver resections can be accomplished by this method, it is considered one of the important techniques in hepatectomy (12,13).
Few groups apply the Glissonian pedicle for open donor hepatectomy (14,15), and only our group have adopted it for PLDH (16). Despite the Glissonian pedicle approach for LLR being challenging, due to restrictions in movement associated with laparoscopic surgery, we believe that the Glissonian pedicle approach for PLDH has several advantages. Firstly, the encircled Glissonian pedicle serves as a landmark during hepatic parenchymal transection. This is an advantage because one of the major disadvantages of LLR is disorientation. The demarcation line, the middle hepatic vein, and the controlled Glissonian pedicle act as good landmarks for guide of hepatic transection. Secondly, because the Glissonian pedicle is thicker and stronger than bile ducts themselves, the Glissonian approach may avoid injuring small bile ducts running from the caudate lobe, which could lead to postoperative bile leakage. Thirdly, minimal dissection around the hepatic duct is possible by the subtraction technique, helping avoid damage to the peribiliary arterial plexus.
Additionally, we dissected the portal pedicle after liver parenchymal transection. This procedure serves a better surgical view during the hepatic hilum dissection. This is achieved because the surgical space was expanded by this step, which separates the right and left lobe. As the Glissonian pedicle dissection is relatively difficult procedure in laparoscopy due to movement restrictions, our strategy of conducting it after liver parenchymal transection might help overcome some of the disadvantages of LLR.
In conclusion, we considered that the Glissonian approach for PLDH can be successfully undergone with good surgical results. Of course, we can not compare outcomes of the Glissonian approach with those of the individual dissection approach. The choice of procedure is determined based on the operator’s preference. Nevertheless, we believe that this novel technique is effective for the treatment of PLDH.
Acknowledgments
We would like to thank the staff of the Department of Anesthesiology, Iwate Medical University School of Medicine for their assistance. We would like to thank Editage (
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kwang-Woong Lee and Jeong-Moo Lee) for the series “Pure Laparoscopic Donor Hepatectomy” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2019.12.07). The series “Pure Laparoscopic Donor Hepatectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Iwate Medical University Institutional Ethics Board (No: MH2018-555), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaneko H, Otsuka Y, Kubota Y, et al. Evolution and revolution of laparoscopic liver resection in Japan. Ann Gastroenterol Surg 2017;1:33-43. [Crossref] [PubMed]
- Egger ME, Gottumukkala V, Wilks JA, et al. Anesthetic and operative considerations for laparoscopic liver resection. Surgery 2017;161:1191-202. [Crossref] [PubMed]
- Ciria R, Ocana S, Gomez-Luque I, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc 2019; [Epub ahead of print]. [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Hasegawa Y, Nitta H, Takahara T, et al. Laparoscopic left hemihepatectomy is suitable as a first step in pure laparoscopic major hepatectomy. Ann Gastroenterol Surg 2018;2:376-82. [Crossref] [PubMed]
- Hasegawa Y, Nitta H, Takahara T, et al. Safely extending the indications of laparoscopic liver resection: When should we start laparoscopic major hepatectomy? Surg Endosc 2017;31:309-16. [Crossref] [PubMed]
- Han HS, Cho JY, Kaneko H, et al. Expert Panel Statement on Laparoscopic Living Donor Hepatectomy. Dig Surg 2018;35:284-8. [Crossref] [PubMed]
- Soubrane O. Laparoscopic Donor Hepatectomy: The Long and Winding Road. Transplantation 2017;101:900-1. [Crossref] [PubMed]
- Kobayashi S, Honda G, Kurata M, et al. An Experimental Study on the Relationship Among Airway Pressure, Pneumoperitoneum Pressure, and Central Venous Pressure in Pure Laparoscopic Hepatectomy. Ann Surg 2016;263:1159-63. [Crossref] [PubMed]
- Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg 1999;16:459-67. [Crossref] [PubMed]
- Takasaki K. Glissonean pedicle transection method for hepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg 1998;5:286-91. [Crossref] [PubMed]
- Yamamoto M, Ariizumi SI. Glissonean pedicle approach in liver surgery. Ann Gastroenterol Surg 2018;2:124-8. [Crossref] [PubMed]
- Sugioka A, Kato Y, Tanahashi Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec's capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci 2017;24:17-23. [Crossref] [PubMed]
- Ikegami T, Shirabe K, Yamashita Y, et al. Small upper midline incision for living donor hemi-liver graft procurement in adults. J Am Coll Surg 2014;219:e39-43. [Crossref] [PubMed]
- Kim SH, Kim YK. Living donor right hepatectomy using the hanging maneuver by Glisson's approach under the upper midline incision. World J Surg 2012;36:401-6. [Crossref] [PubMed]
- Hasegawa Y, Nitta H, Takahara T, et al. Pure laparoscopic living donor hepatectomy using the Glissonean pedicle approach (with video). Surg Endosc 2019;33:2704-9. [Crossref] [PubMed]
Cite this article as: Hasegawa Y, Nitta H, Takahara T, Katagiri H, Kanno S, Umemura A, Sasaki A. Glissonian approach for pure laparoscopic donor hepatectomy. Laparosc Surg 2020;4:11.



