Laparoscopic appendectomy in adult patients: locoregional or general anesthesia? A meta-analysis
Introduction
Acute appendicitis is one of the most common abdominal surgical emergencies worldwide (1), whose standard of care approach is nowadays laparoscopically (2) despite its low to moderate quality of evidences. However, laparoscopic appendectomy (LA) shows some advantages over open surgery, like less wound infections, less postoperative pain, shorter hospital stay and recovery periods for daily activities (3).
The gradual shift towards minimally invasive surgical strategies has dramatically influenced both practices and techniques of anesthesia by propelling innovations and initiatives for newer and safer protocols aiming to early recovery and ambulation (4). Notwithstanding some efforts, laparoscopic surgeries are generally managed under general anesthesia (GA), as a regional one being traditionally widely discouraged due to some concerns about intraoperative discomfort and side effects imbibing empiricism and lack of evidences (5,6). In this regard, although several studies suggest regional anesthesia (RA) may be a safe alternative to GA for laparoscopic surgery (7,8), many aspects remain unsolved, such as the risk of intraoperative hypotension, ventilatory impairment, shoulder pain due to diaphragmatic irritation, patients’ discomfort with subsequent increased surgical time (9). Basing on these discussed and controversial aspects, the feasibility and safety of regional anesthesia in laparoscopic appendectomy had been investigated through a systematic review and meta-analysis by evaluating the pooled intraoperative and postoperative patients’ outcome.
Methods
Study design
A PubMed-MEDLINE Embase, Cochrane Database and Google Scholar literature research was carried out by three investigators from the authors’ group in order to identify relevant data published from Jan 01, 2000 to Oct 31, 2019 on the attempt of a homogeneous report among articles according to availability of techniques and validation of both surgical and anesthesiological expertises. The Boolean function, according the medical subject heading (MeSH) item, was as follows: (((((((laparoscopic) OR minimally invasive) AND appendectomy) AND general anesthesia) OR regional anesthesia) OR epidural anesthesia) OR subarachnoid anesthesia) AND (“2000/01/01”(Date - Publication): “2019/10/31”(Date - Publication)). Additional manual selection process of unlisted references from unindexed sources (Google Scholar) was included via a two-step researches run on November 03, 2019 and November 15, 2019, respectively. All selected potentially relevant papers were reviewed and checked by a four-phase approach (source reliability, title, abstract and critical full-text evaluation) according the following inclusion criteria: (I) both elective and emergent laparoscopic or minimally invasive appendectomy in adult patients; (II) a 1:1 allocated cohort analysis between general and regional anesthetic protocols; (III) a clearly definition of both inclusion and exclusion adopted criteria; (IV) an exhaustive description of surgical techniques (number and sites of lap ports, pneumoperitoneum pressure, medical devices adoption); (V) a detailed explanation of anesthesiological protocols (both for general and regional anesthesia); (VI) a complete description of patients’ outcome according to both intraoperative and postoperative side effects as far as postoperative pain [assessed via a Visual Analogue Scale (VAS) scoring system]; (VII) articles written only in English. Only prospective randomized-controlled trials (RCTs) were included, as the poor statistical relevance of case series, case reports and review articles. Trials comparing different protocols or drug administration in the same cohort were excluded as far as reports about pediatric cases. Subsequent data extraction from eligible articles was carried out by three independent reviewers to collect the following informations: authors’ detail, year of publication, country of publication, type of study, the period of patients’ enrollment, study inclusion and exclusion criteria (if applicable), surgical and anesthetic technique (Table 1).
Table 1
| Author | Year | Country | Type of study | Period | Enrolled patients | Inclusion criteria | Surgical indications | Surgical techniques | Anesthesiological protocols |
|---|---|---|---|---|---|---|---|---|---|
| Erdem MV |
2018 | Turkey | RCT | 2015 | 50 | Adult ASA < III patients, no allergic diathesis, no history of abdominal surgery and pregnancy | Acute appendicitis, carcinoid tumors | Three-port Verres laparoscopy. Trendelenburg position. Ultrasonic-based dissection and endoloop stump closure without any postoperative drainage | No premedication. |
| RA group (combined spinal/epidural anesthesia): hyperbaric bupivacaine 0.5% 10 mg + fentanyl 10 µg L3-L4 injection (spinal); bupivacaine 0.5% 10ml, fentanyl 25 µg and isotonic saline 5 mL (epidural). Level of sensorial nerve block: T4 | |||||||||
| Kumar S |
2019 | India | RCT | 2016 | 100 | Adult ASA < III patients, normal coagulation profile | Acute appendicitis | NR | NR |
| Mehanna AMAM |
2017 | Egypt | RCT | 2015–2016 | 80 | Adult ASA < III patients, BMI < 30 kg/m2 | Acute appendicitis | Three-port open laparoscopy. 12 mmHg CO2 pneumoperitoneum. No trendelenburg position. Ultrasonic-based dissection and endoloop stump closure | GA group: propofol 2.5 mg/kg, fentanyl 1 µg/kg (induction); Atracurium besylate (0.5 mg/gL); Sevoflurane 2–3% with a 0.5 FiO2 oxygen-air mixture |
| RA group: hyperbaric bupivacaine 15 mg + fentanyl 25 µg. Level of sensorial nerve block: T4 | |||||||||
| Shrivastava D |
2019 | India | RCT | 2016–2018 | 50 | Adult ASA < III patients, BMI < 30 kg/m2 | Acute appendicitis | NR | GA group: propofol 2.5 mg/kg, fentanyl 1 µg/kg (induction); Succinyl chlorine 1.5 mg/kg. Atracurium besylate |
| RA group: hyperbaric bupivacaine 15 mg + fentanyl 25 µg. Level of sensorial nerve block: T4 |
RCT, randomized-controlled trial (1:1 allocation); ASA, American Society of Anesthesiologists Classification; VCV, volume-controlled ventilation; NR, not reported.
Endpoints
In order to evaluate both safety and feasibility of regional anesthesia in laparoscopic appendectomy, several primary endpoints were analysed:
- Intraoperative arterial hypotension;
- Intraoperative bradycardia;
- Postoperative shoulder pain;
- Postoperative nausea or vomiting;
- Postoperative bladder retention;
- Postoperative pain.
Secondary endpoint included the evaluation of hospital stay, postoperative mobilization and small bowel sound resumption.
Statistical analysis
The meta-analysis was conducted with Microsoft Excel 2016 (Microsoft®, Redmond, USA) and with IBM SPSS version 20.0 (IBM®, Segrate MI, Italy). Data were reported as absolute numbers (N), percentages (%), means, standard deviations (SDs) with their relative 95% confidence interval (95% CI). Statistical differences or correlations between cohorts were evaluated with paired t-test both for categorical and continuous variables. Standard errors (S.E.) and differences (Df) were also reported. For both primary and secondary endpoints, a summarized 2×2 contingency plot (exposed good event x exposed bad event/ control good event x control bad event) was derived. Relative risk, as defined as the ratio of the probability of an event occurs in an exposed cohort versus the probability the same event occurs in the control one, was calculated according to the formula: RR = [a/(a+b)]/[c/(c+d)]. The overall test effect for each item was assessed according to both z-effect function and P value derivation. A value <0.05 for both was considered significant. RR Forest plots were derived for summarized visual evaluation. A RR threshold >1.00 was considered significant and thus suggesting non-negligible positive or negative effects.
Results
Data extraction process
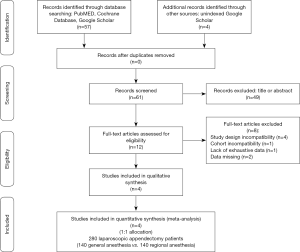
According the MeSH terms Boolean function and throughout the adoption of the PRISMA © flow diagram guidelines (http://www.prisma-statement.org/), 57 potentially relevant articles were identified by four investigators. Further, 4 unindexed papers were included from web research for a total of 61 eligible for analysis. Thereafter, 39 were removed basing upon title and ulterior 10 after their abstract evaluation. A second step full-text analysis was brought out for the 12 remaining articles and only four were included for meta-analysis (10-13). In particular: (I) four papers were rejected due to their study design incompatibility (case series reports); (II) one article due to cohort incompatibility (pediatric population); (III) one article due to the lack of exhaustive data and (IV) two articles due to the availability of only abstracts. At the end of this process, 280 1:1 randomized allocated patients (140 RA-LA vs. 140 GA-LA) were enrolled (Figure 1).
Primary quantitative and qualitative evaluation
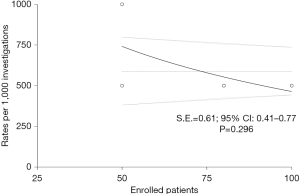
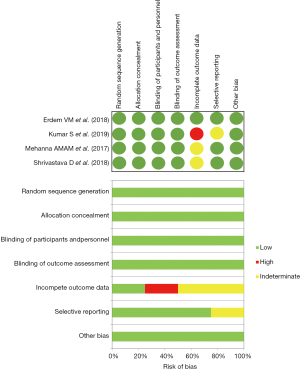
Preliminarily, a quantitative evaluation of the eligible articles was carried out in order to evaluate the presence of any selection bias through an asymmetry funnel plotting test which confirmed the heterogeneity of the enrolled population (S.E.=0.61, 95% CI: 0.41–0.77; P=0.296) (Figure 2). Concerning with qualitative analysis, investigators referred to the Cochrane Collaboration’s tool (14), as reported in Figure 3. Although no critical issues were found in the design of the studies (randomization, allocation, double-blind analysis), sources of bias were highlighted in patients’ outcome. In particular, one study (11) presented an incomplete description of intraoperative side effects and resorted to an arbitrary and inexhaustible assessment of the pain, resulting in a non-negligible risk of selective reporting. Two further articles (12,13), on the other hand, lacked secondary details about the demographic characteristics of the enrolled populations.
Intra- and postoperative events: primary endpoints
Arterial hypotension
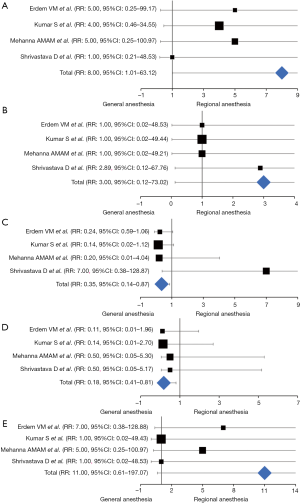
Episodes of intraoperative arterial hypotension were reported in all the elected article, enrolling 280 patients (RA-LA vs. GA-LA: 140 vs. 140). At the weighted-pooled analysis, no significant cumulative effects were found among included studies (17.86% vs. 35.71% vs. 28.57% vs. 17.86%; 95% CI: −11.47–29.25, Df: 10.71, P=0.332). With an incidence of significant decrease in blood pressure (σ>2 SD) of 5.71% and 0.71% respectively, a significant difference between cohorts was found (RR: 8.00, 95% CI: 1.01–63.12, z-effect: 1.97, P=0.048), suggesting an increased risk of intraoperative hypotension events in patients undergoing awake laparoscopic surgery (Table 2) (Figure 4A).
Table 2
| RCTs | N pts | RA-LA | GA-LA | RR | 95%CI | z-effect | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % (mean ± SD) | N | % (mean ± SD) | ||||||||
| Arterial hypotension | ( |
280 | 140 | 5.71 | 140 | 0.71 | 8.00 | 1.01–63.12 | 1.97 | 0.048 | |
| Bradicardia | ( |
280 | 140 | 0.71 | 140 | 0 | 3.00 | 0.12–73.02 | 0.67 | 0.500 | |
| Shoulder pain | ( |
280 | 140 | 2.75 | 140 | 6.37 | 0.35 | 0.14–0.87 | 2.27 | 0.023 | |
| PONV | ( |
280 | 140 | 0.81 | 140 | 4.37 | 0.18 | 0.41–0.80 | 2.24 | 0.024 | |
| POUR | ( |
280 | 140 | 3.57 | 140 | 0 | 11.00 | 0.61–197.07 | 1.63 | 0.103 | |
| Postoperative pain | ( |
280 | 140 | (1.17±0.39) | 140 | (4.83±0.70) | NA | 3.53–3.79 | NA | <0.001 | |
| Hospital stay | ( |
150 | 75 | (1.00±1.15) | 75 | (1.00±1.15) | NA | −0.85–0.85 | NA | 1.000 | |
| Time to mobilization (hours) | ( |
130 | 65 | (11.75±1.75) | 65 | (16.60±2.70) | NA | 4.06–5.64 | NA | <0.001 | |
| Bowel sounds resumption (hours) | ( |
130 | 65 | (6.95±1.50) | 65 | (7.85±2.35) | NA | 0.21–1.58 | NA | 0.011 | |
RA-LA, regional anesthesia laparoscopic appendectomy; GA-LA, general anesthesia laparoscopic appendectomy; RCTs, randomised-controlled trials; PONV, postoperative nausea and vomiting; POUR, postoperative urine retention; SD, standard deviation; RR, relative risk; CI, confidence interval; NA, not applicable.
Bradycardia
The occurrence of bradycardic episodes was investigated in all articles, enrolling 280 patients (RA-LA vs. GA-LA: 140 vs. 140). At the weighted-pooled analysis, no significant cumulative effects were found among included studies (17.86% vs. 35.71% vs. 28.57% vs. 17.86%; 95% CI: −11.47–29.25, Df: 10.71, P=0.332). With a rough incidence of 0.71% and 0%, the Forrest analysis revealed no significant correlations between the anesthetic technique and the occurrence of bradycardic episodes, although in the presence of a risk value consistently above the influence threshold (RR: 3.00, 95% CI: 0.12–73.02, z-effect: 0.67, P=0.500) (Table 2) (Figure 4B).
Shoulder pain
Shoulder pain was described in all the reports. At the weighted-pooled analysis, no significant cumulative effects were found among included studies (17.86% vs. 35.71% vs. 28.57% vs. 17.86%; 95% CI: −11.47–29.25, Df: 10.71, P=0.332). With an incidence of 2.75% and 6.37% between cohorts, patients undergoing RA-LA experienced a substantial reduction of painful episodes without any significant exposure risk than their GA counterparts (RR: 0.35, 95% CI: 0.14–0.87, z-effect: 2.27, P=0.023) (Table 2) (Figure 4C).
Postoperative nausea and vomiting (PONV)
The eventuality of PONV episodes was evaluated in all the RCTs elected. At the weighted-pooled analysis, no significant cumulative effects were found among included studies (17.86% vs. 35.71% vs. 28.57% vs. 17.86%; 95% CI: −11.47–29.25, Df: 10.71, P=0.332) and a PONV incidence of 0.81% and 4.37% was reported. Locoregional anesthesia techniques applied to the laparoscopic approach significantly preserved patients from the aforementioned postoperative side effect (RR: 0.18, 95% CI: 0.41–0.80, z-effect: 2.24, P=0.024) (Table 2) (Figure 4D).
Urinary retention
Postoperative urinary retention (POUR) was evaluated in all the RCTs elected. At the weighted-pooled analysis, no significant cumulative effects were found among included studies (17.86% vs. 35.71% vs. 28.57% vs. 17.86%; 95% CI: −11.47–29.25, Df: 10.71, P=0.332) and a POUR incidence of 3.57% and 0% between cohorts was reported. Although RA-LA patients were significantly exposed to an augmented risk of micturition dyssynergism (RR: 11.00), no significant differences were reported (95% CI: 0.61–197.07, z-effect: 1.63; P=0.103) (Table 2) (Figure 4E).
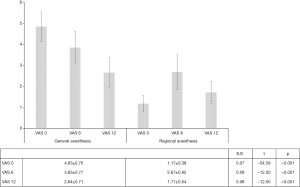
Postoperative pain (visual analogue assessment)
Postoperative pain was assessed by adopting the analogue visual scale, through repeated interviews in the immediate awakening (VAS 0), six hours (VAS 6) and twelve hours after surgery (VAS 12). At each survey, a statistically significant difference between the two groups was noted, consistently favouring a locoregional approach (VAS 0 vs. VAS 6 vs. VAS 12: S.E.=0.07, t=−54.59, P<0.001; S.E.=0.09, t=−12.20, P<0.001 and S.E.=0.08, t=−12.50, P<0.001). In detail, in the immediate perioperative period, RA LA patients reported a pain threshold three-times lower than in patients undergoing the conventional procedure (1.17±0.39 vs. 4.83±0.70, 95% CI: 3.53–3.79, Df: 3.66, S.E.=0.07, t=54.04, P<0.001) (Table 2) (Figure 5).
Patients’ outcome: secondary endpoints
Hospital stay
Postoperative hospital stay was investigated in two of the articles’ panel, enrolling 150 patients (RA-LA vs. GA-LA: 75 vs. 75). At the weighted-pooled analysis, a strong cumulative effect from one study was discovered (33.33% vs. 66.66%; 95% CI: 9.36–52.31, Df: 33.33, P=0.006). For this reason, the analysis failed to demonstrate any superiority or significant difference between techniques, although the comparison did not indicate any substantial disparity between approaches (1.00±1.15 vs. 1.00±1.15, 95% CI: −0.85–0.85, Df: 0.00, S.E.: 0.42, P=1.000) (Table 2).
Time to mobilization
The time to mobilization was reported in two papers for a total of 130 patients (RA-LA vs. GA-LA: 65 vs. 65). Also this time, an influent cumulative effect was reported (33.33% vs. 66.66%; 95% CI: 9.36–52.31, Df: 33.33, P=0.006), leading to abort further evaluations. However, at the t-paired analysis, RA-LA patients experienced an earlier postoperative mobilization than the control group (RA-LA vs. GA-LA: 11.75±1.75 vs. 16.60±2.70; 95% CI: 4.06–5.64, Df: 4.85, S.E.: 0.40, P<0.001) (Table 2).
Bowel sounds resumption
The resumption of postoperative peristaltic activity was investigated by two RCTs (130 patients). Notwithstanding, a similar cumulative effect than previous analyses was confirmed, locoregional anesthesia allowed a rapid bowel sound restoration in the immediate postoperative period (RA-LA vs. GA-LA: 6.95±1.50 vs. 7.85±2.35; 95% CI: 0.21–1.58, Df: 0.90, S.E.: 0.35, P=0.011) (Table 2).
Discussion
Appendicitis is the most common abdominal emergency in all age groups (1), as up 10% of the general population experience such condition (15). Open appendectomy has been the gold standard of treatment for many decades, but the efficiency of a laparoscopic approach has gained popularity though still rises debate (16). However, there is strong evidence that the minimal surgical trauma results in less wound infections, less postoperative pain, shorter hospital stay and recovery periods for daily activities (3). Generally, laparoscopic surgery has dramatically reduced postoperative morbidity (17), advocating new challenges for anesthesiologists, such as the management of the effects of pneumoperitoneum, the risk of intraoperative gas embolism and the balancing of an optimal intra- and postoperative analgesia (18). In this setting, regional anesthesia has not gained popularity, rather remaining in an aura of empiricism. Historically, therefore, the use of general anesthesia with controlled ventilation and maintenance of an end-tidal carbon dioxide (EtCO2) around 35mmHg has always been considered the most acceptable technique during laparoscopic procedures. However, despite a general skepticism, locoregional anesthesia has several advantages upon general anesthesia such as a faster recovery, a reduced risk of emesis, an effective postoperative analgesia, a lower incidence of deep vein thrombosis and the absence of endotracheal intubation with subsequent risks of postoperative atelectasis and pneumonia (19). In literature, on the other hand, rare experiences are published about regional anesthesia in laparoscopic surgery and they are usually relegated to small series of monocentric cases or to cohorts of high-risk patients in which the choice of local techniques appears mandatory (7).
The aim of this meta-analysis was therefore to provide some firm points about the safety and efficacy RA during appendectomy. Preliminarily, it is necessary to clarify that Authors decided excluded cohorts of pediatric patients in order to ensure a homogeneous sample enrollment. The eligible RCTs, therefore, included only adult cases with an absent to moderate comorbidity severity scores (ASA I-II) in order to minimize the effect of a potential patients’ selection bias.
Cardiovascular impairment is one of the major theoretical issue during RA-LA. In fact, results seem to confirm an increased risk of intraoperative hypotensive events (RR: 8,00, P=0.04) as a consequence of decreased peripheral resistances and venous return secondary to sympathetic blockade. A cumulative incidence of 5.71% was observed in the analysis. On the other hand, regional anesthesia techniques do not influence the onset of post-operative bradycardias (0.71%, RR: 3.00, P=0.50), consistently with Mehta et al. (20) who, in a RCT about the efficacy of spinal anesthesia in laparoscopic cholecystectomies, did not report episodes of bradycardia suggesting cardiovascular variations are partially offset by the effects of pneumoperitoneum through the stimulation of the renin-angiotensin-aldosterone system resulting in an increase in cardiac preload secondary to an increased venous return (21). But, the only independent factor related to the severity of hypotension is the level of sensory block (22), as the maximum risk is directly proportional to the maximum ascension threshold of metameric block (10-13,19,23-26).
Concerning with postoperative shoulder pain, the pathophysiological mechanisms and the induced neuropathic stimulus on the phrenic nerve (C3-C5) of pneumoperitoneum and carbon dioxide by laparoscopy are not completely clear. Although it is common practice to counter the late effects of pneumoperitoneum by instilling warm physiology at the end of the procedure with patient in the Trendelenburg position or by forced pulmonary recruitment maneuvers with open trocars, van Dijk et al. (27), in an RCT including 200 patients undergoing gynecological laparoscopies demonstrated clinical ineffectiveness since there were no differences between the intervention and control group both in the immediate perioperative period (38% vs. 50%, P=0.11) and in the first 48 post-operative hours (21% vs. 25%, P=0.57). The controversial aspects of laparoscopy-induced shoulder pain raise further issues as no significant topographic abdominal explorations nor technical surgical procedures significantly influence the intensity of postoperative algia. Donatsky et al. (28), in a systematic review on laparoscopic cholecystectomies, reported the absence of recommendation to undertake abdominal wall lift techniques in order to reduce wall tension. On the other hand, the Authors recognized the effectiveness of a low-pressure pneumoperitoneum as a precaution to minimize the incidence and severity of pain. However, this strategy cannot be pursued in all patients, such as in obese patients where increased insufflation pressures are required (PP: 12–14 mmHg) in order to overcome parietal inertia and ensure a magnified operating field. Furthermore, a reduced pneumoperitoneum (PP: <10 mmHg) would involve technical problems such as the risk of steric hindrance between ports, the impossibility of performing atraumatic gas-induced dissections and the inability to find anatomical landmarks. According to our analysis, the interventional cohort showed a statistical reduction postoperative shoulder pain (2.75% vs. 6.37%. RR: 0.35, P=0.023), suggesting a central role of both prolonged RA-induced analgesia and the lack of a respiratory depression to hypercapnia such as to actively contrast peritoneal hyperinflation.
Postoperative nausea and vomiting (PONV) is one of the significant problems in anesthesia. The pathophysiology of PONV involves several afferent pathways such as chemoreceptor trigger zones, vagal pathways, vestibular systems, midbrain afferents and reflex afferent roots (29). However, there is a wide array of anesthetic, surgical and patients’ factors influencing PONV and its still high incidence. Concerning with regional anesthesia, PONV could result from hypotension episodes, anesthetic mixtures, high-level sensory block as far as from the inherent toxicity of local anesthetics (30). According to results, less than 1% of RA LA patients experienced PONV with a significant difference with GA brace (RR: 0.181, P=0.024) which is consistent with a RA-related reported PONV incidence between 0% and 12.50% (31,32). Concerning with early in-hospital course, the only essential side-effect in the RA cohort was urinary retention (RR: 11.00, P=0.10), confirming regional techniques as risk factors with rates up to 35% in some series (33), as the result of a complex process involving several neural pathways (S2-S3 sacral segments, µ and δ spinal cord receptors), reflexes (detrusor sphincter dissenergy) and central pontine nuclei, where local anesthetics and opioids act (34). But the high relative risk dissonates with a lack of statistical significance. This latter does not seem to be a mere statistical finding as it confirms the depressive action of general anesthetics (muscle relaxants, volatile and sedative-hypnotic anesthetics) on detrusor contraction and micturition reflex.
Further, results of the present analysis revealed VAS score was significantly lower in the postoperative period in RA patients rather than their counterparts (RA-VAS vs. GA-VAS: 1.85±0.58 vs. 3.77±0.73; P<0.001). Gurudatta et al. (35), in a cohort study comparing 50 patients undergoing low abdominal laparoscopic surgery (cystectomy, appendectomy, ovarian drilling) both in GA and RA, reported substantial differences both in the immediate (RA-VAS vs. GA-VAS: 0.10±0.30 vs. 7.10±0.70; P<0.001) and the early postoperative period (2.10±0.08 vs. 5.9±0.70; P=0.009).
Finally, another crucial aspect is postoperative patients’ outcome and time-to-resumption from surgery. Although our analysis failed to demonstrate any evidence about hospital stay due to statistical concerns, raw available data allow to deduce that in the modest examined sample, no significant differences between classic and regional anesthesiological conducts were found. These conclusions, therefore, make indirectly to speculate about both the safety and efficacy of RA in laparoscopic appendicectomies (95% CI: −0.85–0.85, Df: 0.00, S.E.: 0.42; P=1.000), since postoperative hospitalization reflect a primary indicator of morbidity as emerged from the subgroup analysis of primary endpoints. Other confirmations come from the evaluation of secondary aims and in particular from the indisputable benefits of an early mobilization (RA-LA vs. GA-LA: 11.75±1.75 vs. 16.60±2.70, P<0.001) and a prompt resumption of the peristaltic activity (RA-LA vs. GA-LA: 6.95±1.50 vs. 7.85±2.35, P=0.011).
Study limits
The study design was developed based on a rigorous statistical methodology (PRISMA statements, Cochrane Collaboration Tools). Notwithstanding the absence of a population homogeneity (selection bias) that would have invalidated results (95% CI: 0.41–0.77, P=0.296), current meta-analysis presents some limitations. First, the restricted cohort of patients could lead to a type 2 statistical error (β >20%) due to the weight percentage of a RCT (12) in the pooled analysis and second, a RCT (11) lacked an exhaustive methodological report, although a rigorous comparison between anesthetic techniques (RA vs. GA) was declared.
Conclusions
Locoregional anesthesia laparoscopic appendectomies in adult patients ensure a significant reduction in postoperative adverse events of typical general anesthesiological conduct. The indications for surgical intervention (elective or urgent) do not influence the possibility to perform the procedure safely requiring, instead, a synergy of the operating room team both from a technical and methodological point of view. In conclusions, the analysis clarifies the undoubted role of locoregional anesthesia in laparoscopic visceral surgery, as a valid alternative in the general population and no longer as a strategy reserved for high-risk patients. Further dedicated subpopulation and RCT studies are required for pediatric patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2020.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ceresoli M, Zucchi A, Allievi AH. Acute appendicitis: Epidemiology, treatment and outcomes-analysis of 16544 consecutive cases. World J Gastrointest Surg 2016;8:693-9. [Crossref] [PubMed]
- Masoomi H, Mills S, Dolich MO, et al. Comparison of outcomes of laparoscopic versus open appendectomy in adults: data from the Nationwide Inpatient Sample (NIS), 2006-2008. J Gastrointest Surg 2011;15:2226-31. [Crossref] [PubMed]
- Jaschinski T, Mosch CG, Eikermann M, Neugebauer EA, Sauerland S. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001546.pub4/full
- Moningi S, Patki A, Padhy N, et al. Enhanced recovery after surgery: An anesthesiologist’s perspective. J Anaesthesiol Clin Pharmacol 2019;35:S5. [PubMed]
- Bajwa SJS, Kulshrestha A. Anaesthesia for laparoscopic surgery: General vs regional anaesthesia. J Minim Access Surg 2016;12:4. [Crossref] [PubMed]
- Collins LM, Vaghadia H. Regional anesthesia for laparoscopy. Anesthesiol Clin North Am 2001;19:43-55. [Crossref] [PubMed]
- van Zundert AA, Stultiens G, Jakimowicz JJ, et al. Laparoscopic cholecystectomy under segmental thoracic spinal anaesthesia: a feasibility study. Br J Anaesth 2007;98:682-6. [Crossref] [PubMed]
- Jun GW, Kim MS, Yang HJ, et al. Laparoscopic appendectomy under spinal anesthesia with dexmedetomidine infusion. Korean J Anesthesiol 2014;67:246-51. [Crossref] [PubMed]
- Imbelloni LE, Fornasari M, Fialho JC, et al. General anesthesia versus spinal anesthesia for laparoscopic cholecystectomy. Rev Bras Anestesiol 2010;60:217-27. [PubMed]
- Erdem VM, Donmez T, Uzman S, et al. Spinal/epidural block as an alternative to general anesthesia for laparoscopic appendectomy: a prospective randomized clinical study. Wideochir Inne Tech Maloinwazyjne 2018;13:148-56. [PubMed]
- Kumar S, Horo V. A comparative study between spinal vs general anesthesia for laparoscopic appendectomy a randomized controlled trial. Glob J Res Anal 2019;8.
- Mokhtar Mehanna AMA, Ibrahim AG. Comparative Study between General and Spinal Anaesthesia in Laparoscopic Appendectomy. J Anesth Clin Res 2017;08: [Crossref]
- Shrivastava D, Bhadkaria DA. General Anaesthesia vs Spinal Anaesthesia for Laparoscopic Appendectomy; A Comparative Study. Asian J Med Res 2018;7:AN06-AN10.
- The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. Available online: https://www.bmj.com/content/343/bmj.d5928
- Kurtz RJ, Heimann TM. Comparison of open and laparoscopic treatment of acute appendicitis. Am J Surg 2001;182:211-4. [Crossref] [PubMed]
- Biondi A, Grosso G, Mistretta A, et al. Laparoscopic vs. open approach for colorectal cancer: evolution over time of minimal invasive surgery. BMC Surg 2013;13:S12. [Crossref] [PubMed]
- Hendolin HI, Pääkönen ME, Alhava EM, et al. Laparoscopic or open cholecystectomy: a prospective randomised trial to compare postoperative pain, pulmonary function, and stress response. Eur J Surg 2000;166:394-9. [Crossref] [PubMed]
- Salihoglu Z, Demiroluk S, Dikmen Y. Respiratory mechanics in morbid obese patients with chronic obstructive pulmonary disease and hypertension during pneumoperitoneum. Eur J Anaesthesiol 2003;20:658-61. [Crossref] [PubMed]
- Sinha R, Gurwara AK, Gupta SC. Laparoscopic total extraperitoneal inguinal hernia repair under spinal anesthesia: a study of 480 patients. J Laparoendosc Adv Surg Tech A 2008;18:673-7. [Crossref] [PubMed]
- Mehta PJ, Chavda HR, Wadhwana AP, et al. Comparative analysis of spinal versus general anesthesia for laparoscopic cholecystectomy: A controlled, prospective, randomized trial. Anesth Essays Res 2010;4:91-5. [Crossref] [PubMed]
- Gutt CN, Oniu T, Mehrabi A, et al. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg 2004;21:95-105. [Crossref] [PubMed]
- Hartmann B, Junger A, Klasen J, et al. The incidence and risk factors for hypotension after spinal anesthesia induction: an analysis with automated data collection. Anesth Analg 2002;94:1521-9. [PubMed]
- Zhang N, He L, Ni JX. Level of sensory block after spinal anesthesia as a predictor of hypotension in parturient. Medicine (Baltimore) 2017;96:e7184 [Crossref] [PubMed]
- Donmez T, Erdem VM, Uzman S, et al. Laparoscopic cholecystectomy under spinal-epidural anesthesia vs. general anaesthesia: a prospective randomised study. Ann Surg Treat Res 2017;92:136. [Crossref] [PubMed]
- Zacharoulis D, Fafoulakis F, Baloyiannis I, et al. Laparoscopic transabdominal preperitoneal repair of inguinal hernia under spinal anesthesia: a pilot study. Am J Surg 2009;198:456-9. [Crossref] [PubMed]
- Arya RA, Kumar N, Rao ASN. Evaluation of Laparoscopic Total Extraperitoneal Repair of Inguinal Hernia Under Regional Anesthesia: A Prospective Case Series. Hell J Surg 2019;91:80-4. [Crossref]
- van Dijk J, Dedden SJ, Geomini P, et al. Randomised controlled trial to estimate reduction in pain after laparoscopic surgery when using a combination therapy of intraperitoneal normal saline and the pulmonary recruitment manoeuvre. BJOG 2018;125:1469-76. [Crossref] [PubMed]
- Donatsky AM, Bjerrum F, Gögenur I. Surgical techniques to minimize shoulder pain after laparoscopic cholecystectomy. A systematic review. Surg Endosc 2013;27:2275-82. [Crossref] [PubMed]
- Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2014;118:85-113. [Crossref] [PubMed]
- Knudsen K, Beckman Suurküla M, Blomberg S, et al. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth 1997;78:507-14. [Crossref] [PubMed]
- do Amaral PCG, Filho E de MA, Galvão TD, et al. Factors leading to long-term hospitalization after laparoscopic appendectomy. JSLS 2006;10:355-8. [PubMed]
- Uzman S, Donmez T, Erdem VM, et al. Combined spinal-epidural anesthesia in laparoscopic appendectomy: a prospective feasibility study. Ann Surg Treat Res 2017;92:208-13. [Crossref] [PubMed]
- Niazi AA, Taha MA. Postoperative urinary retention after general and spinal anesthesia in orthopedic surgical patients. Egypt J Anaesth 2015;31:65-9. [Crossref]
- Agrawal K, Majhi S, Garg R. Post-operative urinary retention: Review of literature. World J Anesthesiol 2019;8:1-12. [Crossref]
- Gurudatta K, Mohammed A. A Clinical Study of Comparison between General Anesthesia and Spinal Anesthesia for Lower Abdominal Laparoscopic Surgeries. Sch J App Med Sci 2014;1127-33.
Cite this article as: Mucilli F, Barone M, Capone S, Peccianti MM, Guetti L, Prioletta M, Dell’Atti I, Maggiore SM. Laparoscopic appendectomy in adult patients: locoregional or general anesthesia? A meta-analysis. Laparosc Surg 2020;4:26.