
Diagnostic work up and indications for gastro-esophageal reflux surgery
Introduction
Gastro-esophageal reflux disease (GERD) is characterized by the presence of a variety of symptoms induced by the reflux of gastric contents in the esophagus; its estimated prevalence varies between 8% and 33%. The GERD prevalence is 20% in the Western countries and has been increasing over the past 30 years; this increase is attributed to an analogous increase in obesity (1,2).
Pathophysiology of GERD may be explained with the impairment of one or more of the different anatomical and/or motor mechanisms involved in the anti-reflux system (3). Anti-reflux mechanism includes anatomical barriers and esophageal peristalsis. Anti-reflux barrier is a complex region including the intrinsic lower esophageal sphincter (LES), the diaphragmatic crura, the phreno-esophageal ligaments and the acute angle of His. The esophageal peristalsis acts clearing the refluxate to reduce exposure to noxious components of gastric juice (4-7). Increased gastric secretion, and excess of gastric content secondary to delayed gastric emptying may contribute to reflux events (3, 7).
The most common cause of reflux is considered the Transient Lower Esophageal Sphincter Relaxation (TLESR), which are non-swallow-induced relaxations of the LES to enable venting of gas from the stomach. It is still controversial whether subjects with GERD have more TLESRs that are probably induced by the afferent sensory mechanisms of the stomach, involved in a vagal control of LES. It has been reported that TLESRs are not more frequent in patients with GERD than in asymptomatic volunteers, but when TLESRs occur in GERD patients, they are twice as likely to have acid reflux (8).
Lifestyle factors, such as weight gain and high body mass index, high dietary fat intake and carbonated drinks consumption, are recognized to develop or exacerbate GERD symptoms; it is not clear which role alcohol and smoking play in leading to GERD (3). Body weight seems to be an important risk factor; in our experience of 24-hour esophageal pH-monitoring for GERD, pathological reflux is more frequent in overweight patients than in normal weight patients (46% vs. 22%, respectively; personal unpublished data).
GERD presents a broad spectrum of symptoms that may or may not be associated with different severity of esophageal mucosa damage. Diagnosis is based primarily on clinical evaluation, investigating typical and atypical symptoms (9), though testing is possible to obtain a better disease characterization, and to define optimal management, be that Proton Pump Inhibitors (PPI) therapy, ARS or cognitive behavioral therapy (10).
The GERD is classified in erosive and Non-Erosive Disease (NERD). Erosive Esophagitis (EE) is defined by the presence of mucosal breaks on the distal esophagus mucosa; NERD presents the same symptoms of EE, without visible mucosal lesions. Upper endoscopy categorizes EE according to the Los Angeles standardized classification, the focus is on the length and on the extent of esophageal mucosal breaks, and it describes four different grades of EE (11).
The NERD affects about 60% of reflux symptomatic patients and includes different phenotypes, depending on the esophageal acid exposition time (AET) and reflux-symptom association. Ambulatory reflux monitoring detects the presence of abnormal esophageal acid exposure, reflux frequency and symptom association with reflux episodes (2,9) and it can distinguish between NERD and reflux hypersensitive. NERD patients have an abnormal esophageal acid exposure with positive reflux-symptoms association, while in reflux hypersensitive patients, symptoms are triggered by reflux episodes despite normal AET (<6%) (12).
Finally, there is a group of patients complaining heartburn or retrosternal discomfort or pain in absence of evidence that they are induced by gastroesophageal reflux, and in absence of histopathology-based esophageal motility disorders. According to Rome IV Diagnostic Criteria (12), this condition is classified as Functional Heartburn and it represents about 10% of patients with heartburn. This group resembles other patients with GERD in terms of clinical presentation and impact on life quality, but the outcome is not satisfactory with PPI therapy because of the persistence of the symptoms (12,13).
A subgroup of subjects with GERD complains of dysphagia in the absence of organic lesions. Dysphagia has to be considered a symptom of alarm, but it is also described as an atypical symptom of GERD. In our previous observation dysphagia was statistically associated with the presence of hiatal hernia. If dysphagia persists after PPI therapy, further tests are suggested for such patients, as barium esophagus X-ray and/or high-resolution manometry (HRM) to evaluate the presence and the characteristics of the hernia and possible alteration of esophageal motility. In fact, patients with hiatal hernia and GERD symptoms may have just a little benefit from the anti-secretory therapy, due to a delayed esophageal clearance; in these patients, anti-reflux surgery can reach the symptom relief.
Medical treatment
The evaluation of outcome of the medical treatment is a key of selection to candidate the GERD patients to surgery.
Clinicians stress the importance of some lifestyle interventions: weight loss, head of bed elevation, avoiding meals with high fat content and reclining within 2–3 hours after a meal; these recommendations can improve GERD symptoms prior or concurrently with medical therapy, and scientific literature supports this kind of lifestyle habits corrections (9). Esophageal pH-monitoring and GERD symptoms improve from weight loss and head of bed elevation (14-16) and avoiding of late evening meals shows to improve nocturnal gastric acidity (17). Controversial studies have been published about specific dietary interventions (18,19); to date there are no recommendations about broad food elimination (9).
Medical treatment is based on acid suppression and the introduction of the PPI changed the clinical history of GERD and currently they represent the milestone in the treatment of GERD.
Histamine-Receptor Antagonists (H2RA) may be used in alternative to PPI, although studies showed a lower symptoms relief compared to PPI (20). A Cochrane review analyzed 134 treatment trials and found that PPIs reach a better healing effect as well as a faster symptoms relief than H2RA (21). A recent meta-analysis compared PPI, H2RA and prokinetics in GERD symptoms relief and demonstrated PPI as the best treatment (22). The H2RA therapy added to daytime PPI can be offered to patients with inadequately response to PPI and to patients with evidence of night-time reflux, in a bed time administration (23). However, major drawback of H2RA is development of tachyphylaxis (9,24).
Reduction in TLESRs may be achieved by considering vagal afferents as a therapeutic target for GERD; baclofen cannot be used for its severe side effect; emerging similar agents are being investigated (25).
An 8-week course of PPI is the first line medical therapy; symptoms relief and healing of erosive esophagitis are achieved in about 70–80% (9). There are no significant differences in efficacy between different PPIs (23,26). Efficacy of PPI treatment is strongly associated with a relationship between symptoms and reflux. Compared with NERD, symptoms relief with PPI is higher in EE and this may be explained by the presence of other mechanisms than acid occurring in some NERD patients (27,28). However, when NERD patients with proven reflux are analyzed, success rate on symptoms relief is similar to the benefit obtained in patients with EE (29). Therefore, patients who are refractory to PPI need to undergo additional tests to better characterize their disease in order to prove GERD or to detect the acid underlying mechanism. A recent trial evaluated the efficacy of surgical compared to medical treatment among 366 patients with PPI-refractory heartburn (30). A minority of patients experienced relief of heartburn during the 2-week twice daily omeprazole; it’s worth mentioning that patients were instructed to take the medication 20 minutes before breakfast and dinner. After complete evaluation with additional testing, about one third of patients was diagnosed as functional heartburn and only a minority of patients referred with PPI-refractory heartburn underwent reflux surgery. In this selected group success with Nissen fundoplication was significantly larger compared to medical treatment. This study highlights some controversial aspects in management of GERD: (I) the definition of PPI-refractory doesn’t always takes into account correct drug ingestion modality and patient’s compliance; (II) additional tests can be detected non-reflux related disease and other underlying mechanisms may be hypothesized in order to start more appropriate treatment; (III) in highly selected PPI-refractory patients with proven GERD, anti-reflux surgery is superior to medical treatment in relief heartburn.
Additional controversial aspects that may influence the clinician’s decision in the medical treatment of GERD are the management of atypical symptoms and the side effects of long-term PPI therapy. The American GERD guidelines recommend the PPI trial to treat extra-esophageal symptoms in presence of typical GERD symptoms (9). The efficacy of PPI therapy has been demonstrated in this condition (31,32), while there is not much evidence, when typical symptoms are lacking (9).
Response to PPI and reflux monitoring detecting the pathologic presence of gastroesophageal reflux and the association between symptoms and gastro-esophageal reflux are crucial to define an appropriate treatment. It was demonstrated that in the absence of a PPI response, surgery is unlikely to be effective even with abnormal 24-hour pH-impedance findings (33).
It was reported that PPI-test has a good accuracy in predicting reflux etiology (sensitivity 84%, specificity 74%) in patients with non-cardiac chest pain (34), but there are poor data about predicting etiology in patients with other atypical symptoms.
About long-term therapy, PPI therapy maintenance is mandatory in patients with GERD complications including erosive esophagitis and Barrett’s esophagus. In patients with persistence of symptoms after PPI discontinuation, decision of long-term therapy must be cautiously considered, establishing benefit over risk before initiating it (9). In patients with proven GERD by an abnormal pH test, long-term therapy with the lowest dose of PPI can be allowed. In patients with unproven GERD reflux, pH-monitoring is mandatory to define abnormal reflux before long-term PPI therapy starts (23,35). NERD patients with pathological reflux may be managed with on-demand or intermittent PPI therapy (9,36). H2RA or neuromodulators are indicated in patients with reflux hypersensitivity, whereas functional heartburn should be managed with other medical and no medical strategies (i.e. neuromodulators and psychotherapy) (9,23).
When long-term PPI treatment is needed, their adverse effects should be considered and patients should be monitored accordingly. American guidelines recommend only stop of PPI in patients at risk of Clostridium Difficile infection and other enteric infection (9). A recent expert review highlights the importance of patient selection for long-term therapy and suggests to attempt reduction at minimal dose or stop when possible (for example in patients with uncomplicated GERD), with periodically reevaluation. There are some reports about their role on mineral and/or vitamin deficiencies, but in absence of evidence, any their monitoring is not recommended (37).
Long-term PPI treatment frequently does not induce a sufficient benefit in NERD patients; moreover, the chronic assumption of drug may affect the patient’s compliance.
In addition, PPI therapy reduces the symptoms and damage caused by the acid component of the reflux, but does not affect the reflux and the effects of the non-acid components of the reflux (bile, pepsin, etc.). The persistence of the symptoms can be related to the non-acid reflux, and some subjects treated with PPI therapy benefited on heartburn or on other symptoms, but they had to sleep in orthopneic position due to the occurrence of bulky refluxes causing cough and dyspnea when lying. In all these cases it can be necessary changing the plan of treatment.
Surgery: who, when, how?
American GERD guidelines suggest to consider anti-reflux surgery for GERD patients with long-term therapy, or in presence of side-effects associated with medical therapy, or in presence of esophagitis refractory to medical therapy, or if symptoms are persistent, despite long-term PPI therapy (9).
According to ICARUS consensus prior to reflux surgery patients must undergo a complete evaluation: upper gastrointestinal endoscopy, 24-hour pH-impedance monitoring, high resolution manometry. Also, patients with suspicion of hiatus hernia or short esophagus need to undergo a barium swallow, while a gastric emptying test is recommended in patients with dyspeptic symptoms (2).
The successful outcome of surgery depends on the accurate selection of the candidates (30,38).
A group of surgical candidates are PPI non-responders. To assess the lack of response to the PPI it is necessary to investigate the patient’s compliance, the tablet intake timing, the dosage of the PPI and to evaluate the persistence of acid refluxes during PPI treatment (9,23,30,39).
In addition, the definition of PPI refractory is questionable. In scientific literature Authors debate whether the lack of response should be considered after 8 weeks of treatment with a single or a double PPI dose (9,40,41).
Surgical treatment of GERD can be suggested in patients with atypical symptoms, when it is shown that they are associated with gastric reflux and in patients with persistence of symptoms in the presence of hiatal hernia, without short esophagus (2).
Considering the esophageal functional disorders, persistence of symptoms may benefit from surgical treatment in the case of reflux-hypersensitive esophagus, but not in functional heartburn.
In all candidates for anti-reflux surgery it is useful performing the functional assessment of the esophagus by 24-hour esophageal pH-impedance monitoring and HRM.
A recent expert panel proposed various medical or surgical strategies based on the results of different tests and confirmed the importance of evidence of pathological reflux and/or symptoms-reflux association prior to refer to surgery (42).
Twenty four-hour pH-impedance esophageal monitoring
The 24-hour esophageal pH-impedance monitoring is a gold standard for detection and characterization of reflux episodes (10). Monitoring of the intra-esophageal pH and impedance is indicated in patients with incomplete or lack of response to PPI therapy, in candidates to anti- reflux surgery, and for atypical symptoms like cough, frequent belching, and suspected rumination.
The ICARUS guidelines recommend pH-monitoring (± impedance) prior to referral for anti-reflux surgery (2) in order to characterize reflux-disease and to accurately select patients who can achieve better results. Esophageal AET is the main outcome from reflux monitoring and 6% is the diagnosed with pathological acid reflux. Reflux-symptom association can be detected with pH-metry software to determine whether reflux episodes co-occur with symptoms (10).
The pH-impedance monitoring may be used to assess reflux events with both a pH electrode and a series of impedance electrodes. Compared to the previous pH-monitoring, measuring the reflux as presence in the esophagus of pH <4, the impedance monitoring adds the assessment of weakly acidic refluxes (4≤ pH <7), of weakly alkaline refluxes (pH ≥7), of gaseous refluxes, and re-reflux episodes (43). Advantage of impedance over pH monitoring alone is to detect symptoms triggered by non-acid/weak acid reflux or by gas distension (43,44). Conditions taking advantage of the use of pH-impedance monitoring are chronic cough, excessive belching and rumination syndrome (43). Chronic cough often is associated with weakly acid reflux, whereas in patients with excessive belching, impedance monitoring reliably distinguishes between gastric belch episodes and esophageal belching (45-47).
Both tests (pH and pH-impedance monitoring) can be done ‘on’ or ‘off’ PPI therapy, and the choice depends on symptoms and disease history. Lyon consensus recommended reflux monitoring off PPI in instances of unproven GERD and reflux monitoring on PPI in instances of proven GERD. When GERD has been diagnosed by endoscopic finding of erosive esophagitis or by abnormal pH-monitoring in NERD, the test may be performed on PPI therapy to establish reflux-symptoms correlation and/or to assess adequate acid suppression or poor compliance as the mechanism of persisting symptoms. In contrast, patients with unproven GERD and candidates for reflux surgery may be evaluated off PPI to demonstrate baseline AET (10).
A recent study compared medical treatment versus surgical treatment of reflux disease, reporting that a comprehensive evaluation with additional tests can adequately select the patients most likely to experience GERD reduction. The Authors selected patients with abnormal acid reflux detection and/or probability of association of positive symptoms (SAP >95%) on pH monitoring and prescribed a treatment period of 2 weeks with omeprazole 20 mg t.i.d. Patients with partial or absent response were randomized to surgery or active medical treatment or control medical treatment. Active medical treatment was consisted in Omeprazolo 20 mg t.i.d. plus Baclofen and desipramine added sequentially, and control medical treatment was consisted in omeprazolo 20 mg t.i.d. plus Baclofen placebo and desipramine placebo. In this surgery selected population, the benefit at 12 months was achieved in 67% of patients undergoing reflux surgery compared to 28% of patients randomized for active medical therapy (P=0.007) (30).
Esophageal HRM
Esophageal HRM provides functional and anatomical information about body peristalsis, and about gastric-esophageal junction (EGJ) that combines LES and crural diaphragm. It is not recommended in diagnostic assessment of GERD because it is not useful in diagnosis of GERD, but may contribute to explain its pathophysiology. Indeed, there are some functional/motility disorders that may be responsible for GERD symptoms and that need appropriate treatment.
The HRM provides the efficacy of the EGJ with the measurement of the resting pressure; low resting pressure of the EGJ can facilitate the gastric reflux; in our experience we have observed hypotensive LES more frequently in overweight patients than in normal weight patients (46% vs. 16%), and we have detected a trend in decreasing of LES pressure values with increasing of body mass index.
The HRM can describe the disruption of the EGJ or hiatal hernia; in this condition 2 high-pressure zones are detected corresponding to LES (upper) and diaphragmatic crura (lower) respectively (Figure 1). The quantifiable separation between the diaphragmatic crura and LES shows the hernia size. Studies show that HRM has a high sensitivity of 92% and a specificity of 95% for detecting hiatal hernia (48). The Chicago classification describes 3 subtypes of EGJ based on the separation distance between the diaphragmatic crura and the LES: type I, no separation between the LES and diaphragmatic crura; type II, minimal separation (>1 and <2 cm); and type III, ≥2 cm of separation (49).
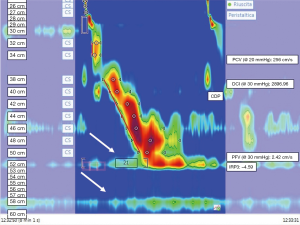
A significant increase in esophageal acid exposure time, and positive reflux-symptom association are seen in association with the type III EGJ morphology (50,51).
Dysphagia is a common complication of ARS, occurring in about 3–24% of patients at six months after surgery (52-55); causes may be related in part to surgical technique. There are different types of wraps that may be used as a possible alternative measure to Nissen fundoplication; it was demonstrated that laparoscopic 270° fundoplication achieves a better outcome than 360° total fundoplication, especially in terms of postoperative dysphagia (56). A systematic review compared results between Toupet fundoplication and Nissen fundoplication showing a higher rate of post-operative dysphagia in the latter one, 8.5% vs. 13.5% respectively (57).
However, dysphagia may also occur because of undiagnosed preexisting motor disorders; in this context, esophageal motility study with HRM is mandatory to select patients for ARS (2). In diagnostic work-up of ARS, HRM enables to detect major esophageal motor disorders that represent a contraindication for surgery (49). Regarding other motor disorders, there are few data supporting that these findings predict post-operative dysphagia. For example, patients with distal esophageal spasm are considered poor candidates for surgery, likely for worsening of symptoms after surgery or appearance of dysphagia. On the contrary, patients with Jackhammer esophagus and previously described Nutcracker esophagus may be considered good candidates for reflux surgery (58); in fact, some retrospective studies have demonstrated no difference in outcomes compared to GERD patients with normal findings on manometry (59).
Contrasting data are reported on surgery outcomes in patients with evidence of Ineffective Esophageal Motility (IEM), which is classified as minor motor disorder involving esophageal peristalsis (60-62).
To date, there are no real contraindications to Nissen because of the lack of data on safety in presence of IEM, but patients and clinicians should be aware of the risk that dysphagia and/or chest pain might occur after surgery. We detected weak peristalsis in 13% of obese patients; although this finding is not clinically significant, this motility abnormality could contribute to GERD physiopathology and should be taken into account when choosing bariatric surgery (sleeve gastrectomy or gastric by-pass) in obese patients.
Provocative testing should be done during manometry in order to assess peristaltic reserve that is suggested as predictor of post-operative dysphagia (23,63). A possible additional testing to improve HRM performance in surgery work-up is Multiple Rapid Swallow (MRS). MRS (Figure 2) consists in repetitive 2 mL volumes administered at 1–2 second intervals with a syringe; repetitive swallowing inhibits esophageal body motility and causes relaxation of the esophago-gastric junction, which is then followed by a clearing contraction (64,65). The lack or weak peristaltic contraction during MRS suggests poor peristaltic reserve and can be associated with dysphagia post-ARS.
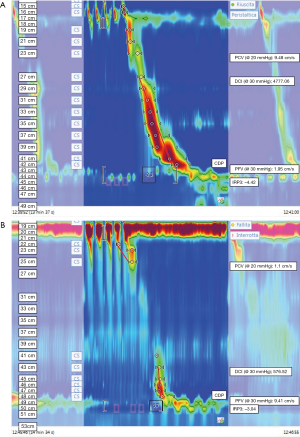
Anisa Shaker et coworkers demonstrated that the patients with postoperative late dysphagia were more likely to have an abnormal MRS than those with no dysphagia. After surgery, patients with late post-operative dysphagia had a significantly pre-operative lower rate of normal MRS than non-dysphagic patients (11.1% vs. 63.6%, respectively P<0.02) (63). Since no large studies have evaluated the role of provocative test, in particular of MRS, more data are necessary to evaluate whether practicing MRS prior to surgery might be useful to tailor ARS.
Conclusions
GERD affects upper GI with a high prevalence all over the world. In a hypothetical diagnostic workup, accurate evaluation of symptoms could be sufficient for diagnosing, but additional tests may be useful to better characterize the disease. Upper endoscopy leads to define an erosive disease, to rule out alternative diagnosis of GERD (for example, eosinophilic esophagitis), to detect long standing GERD complications like Barrett esophagus and adenocarcinoma.
Proton pump inhibitors represent the milestone in the treatment of GERD. When PPI-refractory is suspected, the accurate evaluation of patient’s compliance is necessary and when PPI-refractory is confirmed, additional tests are necessary to detect other underlying mechanisms or overlapping conditions in order to suggest appropriate treatment. If medical treatment is no longer adequate and GERD is proved, anti-reflux surgery is a good strategy after an accurate diagnostic and functional work up for patients’ selection.
Ambulatory reflux monitoring is a gold standard to prove GERD in uncertain conditions and to characterize burden, type of reflux and symptoms-reflux association. Esophageal HRM leads to rule out esophageal motility disorders that may present with the same symptoms, contributing to explain pathophysiology of GERD providing functional information. These additional tests can adequately select the patients most likely to experience benefit from anti-reflux surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Andrea Balla) for the series “Minimally Invasive Approach for the Treatment of Gastro-esophageal Reflux Disease” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-67). The series “Minimally Invasive Approach for the Treatment of Gastro-esophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. DB reports other from VALEAS SPA, Milan, Italy, non-financial support from SOFAR s.p.a. Trezzano Rosa (MI), Italy, personal fees from Messaggi International Publisher & Events, Milan Italy, personal fees from Kyowa Kirin SRL, Milan Italy, personal fees from KardS.r.L, Florence Italy, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Pauwels A, Boecxstaens V, Andrews CN, et al. How to select patients for antireflux surgery? The ICARUS guidelines (international consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery). Gut 2019;68:1928-41. [Crossref] [PubMed]
- Hunt R, Armstrong D, Katelaris P, et al. World Gastroenterology Organization Global Guidelines: GERD Global Perspective on Gastroesophageal Reflux Disease. J Clin Gastroenterol 2017;51:467-78. [Crossref] [PubMed]
- Liu L, Li S, Zhu K, et al. Relationship between esophageal motility and severity of gastroesophageal reflux disease according to the Los Angeles classification. Medicine (Baltimore) 2019;98:e15543 [Crossref] [PubMed]
- Martinucci I, de Bortoli N, Giacchino M, et al. Esophageal motility abnormalities in gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther 2014;5:86-96. [Crossref] [PubMed]
- Savarino E, Gemignani L, Pohl D, et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011;34:476-86. [Crossref] [PubMed]
- Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:267-76. [Crossref] [PubMed]
- Lin S, Li H, Fang X. Esophageal Motor Dysfunctions in Gastroesophageal Reflux Disease and Therapeutic Perspectives. J Neurogastroenterol Motil 2019;25:499-507. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28; quiz 329. [Crossref] [PubMed]
- Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351-62. [Crossref] [PubMed]
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172-80. [Crossref] [PubMed]
- Aziz Q, Fass R, Gyawali CP, et al. Functional Esophageal Disorders. Gastroenterology 2016;150:1368-79. [Crossref] [PubMed]
- Galmiche JP, Clouse RE, Bálint A, et al. Functional esophageal disorders. Gastroenterology 2006;130:1459-65. [Crossref] [PubMed]
- Park SK, Lee T, Yang HJ, et al. Weight loss and waist reduction is associated with improvement in gastroesophageal disease reflux symptoms: A longitudinal study of 15 295 subjects undergoing health checkups. Neurogastroenterol Motil 2017;29: [Crossref] [PubMed]
- Hamilton JW, Boisen RJ, Yamamoto DT, et al. Sleeping on a wedge diminishes exposure of the esophagus to refluxed acid. Dig Dis Sci 1988;33:518-22. [Crossref] [PubMed]
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006;166:965-71. [Crossref] [PubMed]
- Duroux P, Bauerfeind P, Emde C, et al. Early dinner reduces nocturnal gastric acidity. Gut 1989;30:1063-7. [Crossref] [PubMed]
- Piche T, des Varannes SB, Sacher-Huvelin S, et al. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology 2003;124:894-902. [Crossref] [PubMed]
- Festi D, Scaioli E, Baldi F, et al. Body weight, lifestyle, dietary habits and gastroesophageal reflux disease. World J Gastroenterol 2009;15:1690-701. [Crossref] [PubMed]
- Labenz J, Malfertheiner P. Treatment of uncomplicated reflux disease. World J Gastroenterol 2005;11:4291-9. [Crossref] [PubMed]
- Khan M, Santana J, Donnellan C, et al. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;CD003244 [PubMed]
- Sigterman KE, van Pinxteren B, Bonis PA, et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2013;CD002095 [Crossref] [PubMed]
- Gyawali CP, Fass R. Management of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:302-18. [Crossref] [PubMed]
- Fackler WK, Ours TM, Vaezi MF, et al. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002;122:625-32. [Crossref] [PubMed]
- Staunton E, Smid SD, Dent J, et al. Triggering of transient LES relaxations in ferrets: role of sympathetic pathways and effects of baclofen. Am J Physiol Gastrointest Liver Physiol 2000;279:G157-62. [Crossref] [PubMed]
- Gralnek IM, Dulai GS, Fennerty MB, et al. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol 2006;4:1452-8. [Crossref] [PubMed]
- Robinson M, Sahba B, Avner D, et al. A comparison of lansoprazole and ranitidine in the treatment of erosive oesophagitis. Multicentre Investigational Group. Aliment Pharmacol Ther 1995;9:25-31. [Crossref] [PubMed]
- Vantrappen G, Rutgeerts L, Schurmans P, et al. Omeprazole (40 mg) is superior to ranitidine in short-term treatment of ulcerative reflux esophagitis. Dig Dis Sci 1988;33:523-9. [Crossref] [PubMed]
- Weijenborg PW, Cremonini F, Smout AJ, et al. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil 2012;24:747-57, e350.
- Spechler SJ, Hunter JG, Jones KM, et al. Randomized Trial of Medical versus Surgical Treatment for Refractory Heartburn. N Engl J Med 2019;381:1513-23. [Crossref] [PubMed]
- El-Serag HB, Lee P, Buchner A, et al. Lansoprazole treatment of patients with chronic idiopathic laryngitis: a placebo-controlled trial. Am J Gastroenterol 2001;96:979-83. [Crossref] [PubMed]
- Chang AB, Lasserson TJ, Kiljander TO, et al. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 2006;332:11-7. [Crossref] [PubMed]
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006;4:433-41. [Crossref] [PubMed]
- Cremonini F, Wise J, Moayyedi P, et al. Diagnostic and therapeutic use of proton pump inhibitors in non-cardiac chest pain: a meta-analysis. Am J Gastroenterol 2005;100:1226-32. [Crossref] [PubMed]
- Yadlapati R, Kahrilas PJ. When is proton pump inhibitor use appropriate? BMC Med 2017;15:36. [Crossref] [PubMed]
- Pace F, Tonini M, Pallotta S, et al. Systematic review: maintenance treatment of gastro-oesophageal reflux disease with proton pump inhibitors taken 'on-demand'. Aliment Pharmacol Ther 2007;26:195-204. [Crossref] [PubMed]
- Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017;152:706-15. [Crossref] [PubMed]
- Park S, Park JM, Kim JJ, et al. Multicenter Prospective Study of Laparoscopic Nissen Fundoplication for Gastroesophageal Reflux Disease in Korea. J Neurogastroenterol Motil 2019;25:394-402. [Crossref] [PubMed]
- Gunaratnam NT, Jessup TP, Inadomi J, et al. Sub-optimal proton pump inhibitor dosing is prevalent in patients with poorly controlled gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2006;23:1473-7. [Crossref] [PubMed]
- Fass R, Murthy U, Hayden CW, et al. Omeprazole 40 mg once a day is equally effective as lansoprazole 30 mg twice a day in symptom control of patients with gastro-oesophageal reflux disease (GERD) who are resistant to conventional-dose lansoprazole therapy-a prospective, randomized, multi-centre study. Aliment Pharmacol Ther 2000;14:1595-603. [Crossref] [PubMed]
- Fass R, Gasiorowska A. Refractory GERD: what is it? Curr Gastroenterol Rep 2008;10:252-7. [Crossref] [PubMed]
- Yadlapati R, Vaezi MF, Vela MF, et al. Management options for patients with GERD and persistent symptoms on proton pump inhibitors: recommendations from an expert panel. Am J Gastroenterol 2018;113:980-986. [Crossref] [PubMed]
- Roman S, Gyawali CP, Savarino E, et al. GERD consensus group. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017;29:1-15. [Crossref] [PubMed]
- Ravi K, Katzka DA. Esophageal Impedance Monitoring: Clinical Pearls and Pitfalls. Am J Gastroenterol 2016;111:1245-56. [Crossref] [PubMed]
- Blondeau K, Dupont LJ, Mertens V, et al. Improved diagnosis of gastro-oesophageal reflux in patients with unexplained chronic cough. Aliment Pharmacol Ther 2007;25:723-32. [Crossref] [PubMed]
- Ribolsi M, Savarino E, De Bortoli N, et al. Reflux pattern and role of impedance- pH variables in predicting PPI response in patients with suspected GERD- related chronic cough. Aliment Pharmacol Ther 2014;40:966-73. [Crossref] [PubMed]
- Kessing BF, Bredenoord AJ, Smout AJ. The pathophysiology, diagnosis and treatment of excessive belching symptoms. Am J Gastroenterol 2014;109:1196-203; Quiz 1204. [Crossref] [PubMed]
- Weijenborg PW, van Hoeij FB, Smout AJ, et al. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol Motil 2015;27:293-9. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox MInternational High Resolution Manometry Working Group, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Tolone S, de Cassan C, de Bortoli N, et al. Esophagogastric junction morphology is associated with a positive impedance-pH monitoring in patients with GERD. Neurogastroenterol Motil 2015;27:1175-82. [Crossref] [PubMed]
- Ham H, Cho YK, Lee HH, et al. Esophagogastric junction contractile integral and morphology: Two high-resolution manometry metrics of the anti-reflux barrier. J Gastroenterol Hepatol 2017;32:1443-9. [Crossref] [PubMed]
- Sato K, Awad ZT, Filipi CJ, et al. Causes of long-term dysphagia after laparoscopic Nissen fundoplication. JSLS 2002;6:35-40. [PubMed]
- Alexander HC, Hendler RS, Seymour NE, et al. Laparoscopic treatment of gastroesophageal reflux disease. Am Surg 1997;63:434-40. [PubMed]
- Anvari M, Allen CJ. Prospective evaluation of dysphagia before and after laparoscopic Nissen fundoplication without routine division of short gastrics. Surg Laparosc Endosc 1996;6:424-9. [Crossref] [PubMed]
- Eshraghi N, Farahmand M, Soot SJ, et al. Comparison of outcomes of open versus laparoscopic Nissen fundoplication performed in a single practice. Am J Surg 1998;175:371-4. [Crossref] [PubMed]
- Andreou A, Watson DI, Mavridis D, et al. Assessing the efficacy and safety of laparoscopic antireflux procedures for the management of gastroesophageal reflux disease: a systematic review with network meta-analysis. Surg Endosc 2020;34:510-20. [Crossref] [PubMed]
- Broeders JA, Mauritz FA, Ahmed Ali U, et al. Systematic review and meta-analysis of laparoscopic Nissen (posterior total) versus Toupet (posterior partial) fundoplication for gastro-oesophageal reflux disease. Br J Surg 2010;97:1318-30. [Crossref] [PubMed]
- Barreca M, Oelschlager BK, Pellegrini CA. Outcomes of Laparoscopic Nissen Fundoplication in Patients With the "Hypercontractile Esophagus". Arch Surg 2002;137:724-8. [Crossref] [PubMed]
- Dell'Acqua-Cassão B, Mardiros-Herbella FA, Farah JF, et al. Outcomes of laparoscopic Nissen fundoplication in patients with manometric patterns of esophageal motility disorders. Am Surg 2013;79:361-5. [Crossref] [PubMed]
- Gyawali CP, Sifrim D, Carlson DA, et al. Ineffective esophageal motility: Concepts, future directions, and conclusions from the Stanford 2018 symposium. Neurogastroenterol Motil 2019;31:e13584 [Crossref] [PubMed]
- Booth MI, Stratford J, Jones L, et al. Randomized clinical trial of laparoscopic total (Nissen) versus posterior partial (Toupet) fundoplication for gastro-oesophageal reflux disease based on preoperative oesophageal manometry. Br J Surg 2008;95:57-63. [Crossref] [PubMed]
- Mello MD, Shriver AR, Li Y, et al. Ineffective esophageal motility phenotypes following fundoplication in gastroesophageal reflux disease. Neurogastroenterol Motil 2016;28:292-8. [Crossref] [PubMed]
- Shaker A, Stoikes N, Drapekin J, et al. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol 2013;108:1706-12. [Crossref] [PubMed]
- Trudgill NJ, Sifrim D, Sweis R, et al. British Society of Gastroenterology guidelines for oesophageal manometry and oesophageal reflux monitoring. Gut 2019;68:1731-50. [Crossref] [PubMed]
- Fornari F, Bravi I, Penagini R, et al. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil 2009;21:718-e41. [Crossref] [PubMed]
Cite this article as: Sacchi MC, Ribichini E, Severi C, Badiali D. Diagnostic work up and indications for gastro-esophageal reflux surgery. Laparosc Surg 2021;5:23.


