
Robotic resection in liver malignancies
Introduction
The development of minimally invasive procedures has turned over a new leaf in surgery. Since the first laparoscopic cholecystectomy reported in 1988, it has been quickly adopted within few years for its evident benefits. Subsequently, laparoscopic surgery was applied to other fields such as upper gastrointestinal and colorectal procedures. Liver resection is one of the most complex procedures in the abdominal surgery because of the complex vascular and biliary anatomy of liver and perceived risks of bleeding when performing liver parenchymal transection. It is, as no surprise, remained an area of resistance for several years with much slower adoption than other laparoscopic procedures.
The first laparoscopic liver resection was reported in 1992 by Gagner et al. (1). Since then, an increasing number of publications have demonstrated the feasibility and safety of laparoscopic hepatectomy with better perioperative outcomes such as less blood loss, decreased wound pain, better cosmesis, shorter hospital stay, and the most important result: non-inferior oncological outcomes (2-4). Despite of the gradual accumulation evidence of laparoscopic liver resection, it remains as a difficult procedure with steep learning curve which poses challenges for surgeons thus hindering prevalence of this advanced technique. To be competent in laparoscopic liver resection, surgeons must not only be familiar with the basic skills and knowledge of liver surgery, but also advanced laparoscopic techniques including hand-eye coordination under 2-dimentional image in restrictive operation space, straight rigid instruments manipulation, precise liver parenchymal dissection with carefully bleeding control and intra-corporeal suture skills in difficult angles. The rise of robotic system offers a better solution to the limitations mentioned above by better optic visualization and increased freedom of instruments articulation. Its featured EndoWrist instruments provide seven-degrees of freedom, tremor filter and motion scaling which could more accurately replicate surgeons’ wrist and finger movements. The videoscope held by robotic arm, controlled directly by surgeon, offers steady10-fold magnification and 3-dimentional (3D) image. These features allow for precise dissection and better suturing, thus expanding the complexity of procedures. Furthermore, surgeons seated in console instead of standing beside the patient in conventional open surgery, the fatigue caused by prolong operation time in hepatobiliary surgery is also diminished substantially.
The first robotic-assisted liver resection was published by Ryska et al. in 2006 (5). Despite of all the advantages offered by robotic system, the adaptation of robotic liver resection is still slower than other robotic abdominal surgery. This is because of the complexity of minimally invasive liver resection and surgeons need to excel in laparoscopic skills and the manipulation of the robotic system.
This article aimed at reviewing the current status of robotic liver resection, the advantages and disadvantages comparing between laparoscopic and robotic hepatectomy and the learning process of robotic liver resections.
Methods
Literatures search was performed in PubMed and MEDLINE databases using the keywords “robotic liver resection”, “robotic hepatectomy”, “learning curve” and “robotic hepatic surgery”. The data was carefully reviewed to exclude the duplication of cases. Five major series (6-10) were collected and summarized in Table 1.
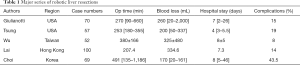
Full table
Robotic versus laparoscopic liver resection
The second international consensus conference of minimally invasive liver resection was held in Morioka Japan in 2014. A study group focusing the issue of robotic liver resection carefully reviewed the published literature and drew some conclusions (11). Currently there was no randomized controlled trials available in terms of comparison between laparoscopic and robotic liver resection. Nevertheless, seventeen major series were analyzed and yielded the feasibility and safety of robotic liver resection with zero mortality and comparable morbidity rates compared with laparoscopic liver resections. Robotic group had a significant higher proportion of major hepatectomies and resection of tumors located at posterosuperior segments compared with laparoscopic group (12,13). That also attributes to longer operative time in robotic groups because surgeons prefer robotic resection in difficult cases. Nota et al. investigated robot-assisted liver resections for three subgroups of liver resection: (I) minor resections of easily accessible segments [2,3,4b,5,6], (II) minor resections of tumors in difficult locations [1,4a,7,8], (III) major hepatectomies and concluded that the robotic platform is safe and suitable in all subgroups of liver resection (13). Theoretically, robotic technology has potential advantages to overcome the obstacles encountered in laparoscopic surgery. However, these are not translated into better perioperative outcomes since comparative studies currently do not show significant difference such as blood loss, R0 resection rate, operation time, morbidity and mortality rate and hospital stay between the two groups.
Liver hilum dissection and parenchymal transection
Liver hilum dissection is one of the pivotal steps in liver surgery, especially in major resections, i.e., right or left hemihepatectomy. It is important to isolate the right/left hepatic artery and portal vein individually or encircle the right/left Glissonean pedicle to control the hepatic inflow before liver parenchymal transection. The wristed robotic instruments which is steady with tremor filter helps to perform dissection around the vasculatures more precise than laparoscopic approach. Surgeons could perform suturing and ligating by robotic instruments easier and better as well that increase the surgeons’ confidence when doing liver hilum dissection. Liver parenchymal transection is critical in both open and minimally invasive liver resection, but it’s more complex in the latter. An experienced bedside assistant is important in robotic liver resection to utilize the suction-irrigation instrument properly which is essential to clean the operation field but it interferes simultaneously with the pneumoperitoneum pressure which is crucial to contain bleeding from veins. When bleeding occurs, the bedside assistant has to be familiar with the techniques of gentle and intermittent suction just enough to identify the bleeder, which allows the console surgeons to manage the bleeding. The common techniques used to control hemorrhage include cauterization, clipping and suture ligation. Hemostasis by suturing is more challenging in laparoscopic approach compared to open surgery. The high definition magnified 3D image in robotic system allows surgeons to identify the bleeding vessels more accurately and the EndoWrist instruments with 7-degrees freedom facilitate intra-corporeal suture better, quicker and easier than laparoscopic approach.
Energy devices are commonly used in liver resection, whether open or minimally hepatectomy. There is a diversity of choices in laparoscopic liver resection but only two systems: harmonic shears and vessel sealant are available in the robotic system and both systems have its own limitation. The harmonic shears have no EndoWrist articulation which limit the angles of dissection while vessel sealant is not ideal for precise dissection due to its bulky sealing facet. These energy devices are usually effective for transecting the superficial layer of liver parenchyma because there are no significant vessels and biliary ducts. For dissection of deeper parenchyma, ultrasonic aspirator [cavitron ultrasonic surgical aspirator (CUSA)] is usually recommended. However, CUSA is not available in the robotic system. Either an experienced bedside assistant to operate the CUSA is needed or the console surgeon has to be comfortable and familiar to perform liver parenchymal transection by using only harmonic shears or vessel sealant. Scissors and hook electrocautery are also usually used in robotic liver resection but the lack of proper sealing ability prevent its prevalent use.
Robotic liver resection of posterosuperior segments
For varies types of hepatectomy including posterosuperior segments resection, the indication and degree of difficulty are not different between laparoscopic and robotic approach. Tumors located in peripheral segments [2,3,4b,5,6] are most amenable to laparoscopic resection. However, resection of those at posterosuperior segments [1,4a,7,8] are considered particularly challenging due to the curved transection lines. The difficulty then leads to more major hepatectomy that sacrificing a substantial volume of normal liver for posterosuperior resections. Ideally, isolated resection of these segments serves patients most benefits but may not be easily achievable. The common technical struggles for minimally invasive resection in posterosuperior segments are poor laparoscopic view, multiplanar parenchymal dissection, narrow space of the subphrenic area and difficult to control bleeding. The features of robotic system may aid to overcome the limits aforementioned.
For resection of posterior segment 6 and 7, complete mobilization of right lobe liver is crucial to create sufficient surgical working space and adequately expose the tumor. The traction by the third robotic arm controlled by surgeon him/herself helps to maintain stability in the working area in the posterior aspect of right liver. Furthermore, the wristed instruments also facilitate the dissection along the convex diaphragm. As for superior central segments [4a,8] resection, the direction of liver parenchymal dissection could be either horizontally or ventral-to-dorsal owing to the advantage of wristed instruments.
One critical step during resection of posterosuperior segments is the major hepatic veins and vena cava. The high definition 3D videoscopic view and wristed instruments of robotic system help surgeons work more confidently and precisely. Even if bleeding occurs, surgeons could apply steady and delicate suture to control bleeding without hesitation.
Nota et al. reviewed 12 studies and suggested that the robotic platform may be of particular advantage in resections of the posterosuperior segments. In addition, Montalti et al. published a propensity score-matched comparison between robotic and laparoscopic resections of posterosuperior segments of the liver (14). They found out a higher rate of parenchymal-preserving resection in posterosuperior segments with the robotic techniques. Robotic assistance could increase the rate of minimally invasive resections especially in cases that required several and multiplanar transection lines.
ICG fluorescence enhancing images
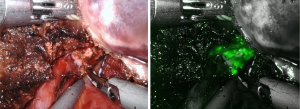
One additional advantage the robotic system has to offer is the indocyanine green (ICG) fluorescence enhancing images by Firefly technique. It enables surgeon to identify the biliary structure, to detect and localize superficial tumors (Figure 1) and to show the clear demarcation boundary between segments. For negative stain method, after the hepatic inflow pedicles were identified, looped and ligated, ICG solution (1 mL/ 2.5 mg) was then injected intravenously. The fluorescence glowing under Firefly mode of robotic system highlighted clear boundary between the normal perfused lobes and dark ischemic segments (Figure 2). For positive stain method, direct puncture with a thin needle into the portal vein supplying the target segments are performed under ultrasonic guided. Diluted ICG solution (50–100 times dilution, 0.05–0.025 mg/mL) was injected into portal vein slowly. The target tumor bearing segments would be shinning and clear seen under robotic Firefly mode. With the aid of ICG-enhancing image, surgeons can proceed precise transection plane and remove all ischemic liver area accordingly. Cho et al. reported a study reveals more complications and longer hospital stay in patients with remnant liver ischemia after surgery (15). Seemingly, higher recurrence rate and lower disease-free and overall survival rates are also noted in patients with remnant ischemic liver. It is thus recommended to perform anatomical resection for liver malignancies and remove all ischemic liver part to achieve better oncological outcomes.

Cost
It’s obvious that robotic system is more expansive than laparoscopic system in terms of the machine, the maintenance fees and the surgical instrumental expanse. Reports (16-18) discussing on cost associated with robotic liver resection revealed mostly higher costs. Nonetheless, the higher costs of robotic surgery didn’t correlate to better outcomes. It is crucial to select proper patients for robotic approach to ensure ideal balance between cost and benefit. Moreover, larger series is warranted to evaluate the benefit of the robotic procedures against the inherent costs.
The future application of robotic system and its clinical value will highly depend on the advantages it can provide over laparoscopic approach, the expanse discrepancy and surgeons experience.
Learning curve
Owing to the limited data on learning curve of robotic liver resection, we could take that of laparoscopic liver resection as reference. According to those studies on the learning curve of laparoscopic hepatectomy (19-23). the minimal case number of minor and major liver resection to suffice the learning curve are 22–64 cases and 45–75 cases respectively. One of the proposed advantages of robotic surgery is the possibility of shortened learning curve for minimally invasive liver resection based on the study of pancreatic surgery (24) which is normally considered equally as complex as liver resection. The robotic system is designed to overcome these difficulties encountered in laparoscopic approach and to optimize conditions in minimally invasive surgery. The learning period of liver resection in robotic system should be shorter compared to laparoscopic group intuitively. Nevertheless, the study regarding the learning curve of robotic liver resection is scarce. Tsung et al. analyzed the impact of learning on robotic hepatectomy (7) which comparing the early (n=13) and later period (n=44). The perioperative outcomes are significant better in the latter group: less blood loss, shorter operation and overall room time, and shorter hospital stay.
One of the major concerns of the robotic system is the lack of tactile feedback and it could lead to collateral injury of visceral organs and breakdown of suture unknowingly. To overcome the disadvantages, with the aid of magnified 3D image, surgeons could “see” the tension applied to tissue and sutures and translate the visual feedback into tactile sensation.
Robotic surgery for hepatic malignancies
The most common indications for hepatic malignancy resections are hepatocellular carcinoma and colorectal liver metastases. The resections with wider margins or anatomical resections show a better oncological outcome compared to atypical resections. The amount of blood loss was also considered as a significant prognostic factor of hepatectomies for malignancies. In the study published by Tsung et al. comparing laparoscopic and robotic hepatectomy (7), a trend towards less blood loss in the robotic major hepatectomies was noted and it could be attributed to magnified optics allowing better identification of vessels during parenchymal transection and efficient bleeding control. In major liver resection, use of robotic platform leads to a larger number of procedures performed by minimally invasive approach and with lower conversion rate.
The management of liver hilar cholangiocarcinoma has always been a thorny issue for surgeons especially in minimally invasive approach. The surgical procedures for hilar cholangiocarcinoma involve liver resection including caudate lobectomy, excision of bile ducts, extensive lymph node (LN) dissection and biliary-enteral anastomosis and each procedure is a tricky challenge for surgeons. The features of precision, dexterity and stability offered by robotic system emerged as the solution for the minimally invasive resection for resection of hilar cholangiocarcinoma. Xu et al. present a series of robotic radical resection for hilar cholangiocarcinoma (n=10) with analysis of perioperative and long-term outcomes compared with conventional open surgery (16). The surgical procedures involve hemi-hepatectomy plus caudate lobectomy or trisegmentectomy, extrahepatic bile duct resection, radical lymphadenectomy and Roux-en-Y hepaticojejunostomy. The operation time was longer (703 vs. 475 min) with higher morbidity rate (90% vs. 50%) in robotic group. The recurrence free survival was inferior in robotic group. The authors concluded that fully robotic radical resection for hilar cholangiocarcinoma is technically achievable but adequate learning is needed for a comparable outcome. Larger series is needed to justify the potential applications of robotic system in hepatic hilar cholangiocarcinoma.
Conclusions
Robotic resection for hepatic malignancies is feasible and safe with comparable oncological outcomes in experience hands. The essential elements for a matured robotic liver surgery program require a dedicated team proficient in equipment, surgical expertise and well-established proctorship. The advantages of robotic system imbue surgeons with precise dissection, steady suture and propel to more complex surgery. As for the higher cost of robotic system, careful patient selection is crucial for better cost-benefit balance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giammauro Berardi) for the series “Minimally Invasive Resections for Liver Malignancies: Among Certainties and Controversies” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at available at http://dx.doi.org/10.21037/ls-20-58). The series “Minimally Invasive Resections for Liver Malignancies: Among Certainties and Controversies” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc 1992;6:97-8.
- Dagher I, Belli G, Fantini C, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg 2010;211:16-23. [Crossref] [PubMed]
- Viganò L, Tayar C, Laurent A, et al. Laparoscopic liver resection: a systematic review. J Hepatobiliary Pancreat Surg 2009;16:410-21. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection—2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Ryska M, Fronek J, Rudis J, et al. Manual and robotic laparoscopic liver resection. Two case-reviews Rozhl Chir 2006;85:511-6. [in Czech]. [PubMed]
- Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: Results for 70 resections. Surgery 2011;149:29-39. [Crossref] [PubMed]
- Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55. [Crossref] [PubMed]
- Wu YM, Hu RH, Lai HS, et al. Robotic-assisted minimally invasive liver resection. Asian J Surg 2014;37:53-7. [Crossref] [PubMed]
- Lai EC, Tang CN. Long-term Survival Analysis of Robotic Versus Conventional Laparoscopic Hepatectomy for Hepatocellular Carcinoma: A Comparative Study. Surg Laparosc Endosc Percutan Tech 2016;26:162-6. [Crossref] [PubMed]
- Choi GH, Chong JU, Han DH, et al. Robotic hepatectomy: the Korean experience and perspective. HepatoBiliary Surg Nutr 2017;6:230-8. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendation for laparoscopic liver resection: a report from the second international consensus conference help in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Lai EC, Yang GP, Tang CN. Robotic-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg 2013;205:697-702. [Crossref] [PubMed]
- Nota CL, Rinkes IB, Molenaar IQ, et al. Robotic-assisted laparoscopic liver resection: a systemic review and pooled analysis of minor and major hepatectomies. HPB 2016;18:113-20. [Crossref] [PubMed]
- Montalti R, Scuderi V, Patriti A, et al. Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc 2016;30:1004-13. [Crossref] [PubMed]
- Cho JY, Han HS, Choi YR, et al. Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma. JAMA Surg 2017;152:386-92. [Crossref] [PubMed]
- Xu Y, Wang HG, Ji WB, et al. Robotic radical resection for hilar cholangiocarcinoma: perioperative and long-term outcomes of an initial series. Surg Endosc 2016;30:3060-70. [Crossref] [PubMed]
- Ji WB, Wang HG, Zhao ZM, et al. Robotic-assisted laparoscopic anatomic hepatectomy in China: Initial experience. Ann Surg 2011;253:342-8. [Crossref] [PubMed]
- Yu YD, Kim KH, Jung DH, et al. Robotic versus laparoscopic liver resection: A comparative study from a single center. Langenbecks Arch Surg 2014;399:1039-45. [Crossref] [PubMed]
- Vigano L, Laurent A, Tayar C, et al. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 2009;250:772-82. [Crossref] [PubMed]
- Lin CW, Tsai TJ, Cheng TY, et al. The learning curve of laparoscopic liver resection after the Louisville statement 2008: Will it be more effective and smooth? Surg Endosc 2016;30:2895-903. [Crossref] [PubMed]
- Cai X, Li Z, Zhang Y, et al. Laparoscopic liver resection and the learning curve: a 14-year, single-center experience. Surg Endosc 2014;28:1334-41. [Crossref] [PubMed]
- Lee W, Woo JW, Lee JK, et al. Comparison of Learning Curves for Major and Minor Laparoscopic Liver Resection. J Laparoendosc Adv Surg Tech A 2016;26:457-64. [Crossref] [PubMed]
- Nomi T, Fuks D, Kawaguchi Y, et al. Learning curve for laparoscopic major hepatectomy. Br J Surg 2015;102:796-804. [Crossref] [PubMed]
- Zeh HJ, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg 2011;45:323-40. [Crossref] [PubMed]
Cite this article as: Lin CCW. Robotic resection in liver malignancies. Laparosc Surg 2021;5:38.


