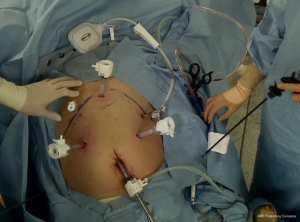
Standard minimally invasive pancreaticoduodenectomy
Introduction
Laparoscopic pancreaticoduodenectomy (LPD) is a technically demanding operation due to high morbidity, complex anatomy, technically challenging anastomosis and relatively rare indications. Since the first report by Gagner and Pomp in 1994 (1), LPD has been adopted more slowly compared with other types of gastrointestinal surgery. With reports of encouraging outcomes by some pioneers (2,3), the reports of LPDs have increased in number. Currently, the number of surgeons performing LPD worldwide is continuing to grow.
A recent systematic review including 224 patients from three randomized controlled trials comparing laparoscopic and open pancreaticoduodenectomy reported no difference in 90-day mortality, complications, or oncologic outcomes. The operation time was significantly longer in the LPD group, but blood loss was significantly less in the LPD group (4-7). Despite several systematic reviews and meta-analyses showing favorable outcomes of LPD, the penetration rate of LPD is still very low due to a long learning curve related to technical difficulty and concerns regarding high morbidity. Therefore, to safely increase the penetration rate of LPD, the complex surgical technique should be standardized and efforts should be made to decrease unfavorable outcomes. Here we introduce our detailed methods of LPD (Video 1), which was established to overcome technical difficulties and reduce operative complications during the learning curve.
Surgical technique
OR setting, device, trocar placement
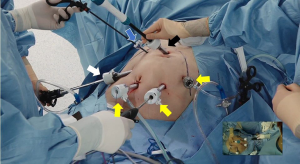
Under general anesthesia, the patient is placed in the lithotomy position, and the reverse Trendelenburg position. After creation of CO2 pneumoperitoneum via a 12-mm infraumbilical port, five additional trocars are placed: four trocars (two 12-mm and two 5-mm) on both sides of the upper abdomen in a curvilinear shape with the infraumbilical trocar in the center, and one 12-mm trocar in the epigastrium (Figure 1).
A 3-D flexible scope (ENDOEYE FLEX 3D, Olympus, Tokyo, Japan) is used. The articulating tip is beneficial in showing a wide range of the operative field, and 3-D visualization can improve precision of surgical tasks by providing depth perception. Although appropriate energy device selection is a crucial key to a successful operation, it depends on personal preference. We frequently use the LigaSure vessel sealing system (Medtronics, Minneapolis, MN, USA) in many ways, from blunt dissection to sealing of small vessels, to hemostasis. In situations where sharp dissection is needed or when the pancreas is transected, the ultrasonic shear (Harmonic scalpel, Ethicon, Somerville, NJ, USA) is useful.
Resection
Division of the gastrocolic ligament
At the beginning of the resection phase, the operator stands on the right side of the patient, the assistant on the left side of the patient, and the scopist between both legs of the patient. The lesser sac is entered by dividing the gastrocolic ligament. Care is taken not to damage the gastroepiploic vessels. While the gastrocolic ligament is divided from the midline towards the duodenum, the posterior side of the stomach is fully mobilized from the anterior side of the pancreas body.
Division of the duodenum
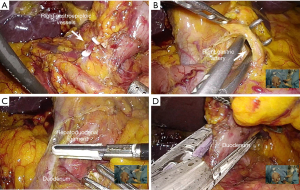
After division of the gastrocolic ligament, the right gastroepiploic vessels are dissected with upper traction of the stomach by the assistant. They are divided about as distal as the resection level of the duodenum (Figure 2A). When the pylorus is preserved, division of the vessels at a level that is too proximal can cause ischemia of the duodenum. As the duodenum is retracted downward to better visualize the base of the hepatoduodenal ligament, the right gastric artery is dissected and divided (Figure 2B). After further dissection of the duodenum from the hepatoduodenal ligament (Figure 2C), the duodenum is transected 2–3 cm distal to the pylorus using an endoscopic linear stapler, which is inserted into the right lower 12 mm trocar (Figure 2D). For this procedure, the assistant needs to lift up the stomach so both jaws of the stapler are visible at the far side of the duodenum to be divided. If the duodenum or lymph nodes around the pylorus are involved, Whipple's procedure is performed.
Kocher maneuver
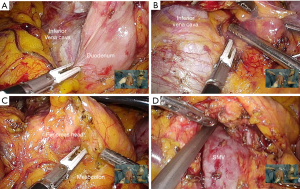
The patient is tilted to the right-up position. As the assistant retracts the duodenal stump to the left side of the patient, the hepatoduodenal ligament and second portion of the duodenum is freed from the anterior surface of the inferior vena cava. Dissection is performed as close to the duodenum as possible, to avoid injury to the right gonadal vein. When performing the inferior aspect of the Kocher maneuver, the third portion of the duodenum is accessed as the assistant applies inferior traction on the mesocolon and the operator lifts up the duodenum (Figure 3A). When there is tension during downward traction of the mesocolon, the hepatic flexure and transverse colon is mobilized. Further mobilization of the distal part of the duodenum is performed until reaching the duodenojejunal junction. Full Kocherization exposing the left renal vein and aorta (Figure 3B) facilitates easier lymph node dissection around the common hepatic artery (CHA) and superior mesenteric artery (SMA), to be performed later.
Approach to the superior mesenteric vein (SMV)
After Kocherization, the anterior aspect of the duodenum and pancreas head are freed from the mesocolon in a right to left direction. Small vessel branches communicating the pancreas head and mesocolon, if present, are controlled using LigaSure. Thereafter, careful dissection is performed along the inferior border of the pancreas to expose the anterior surface of the SMV (Figure 3C). When the SMV is exposed, the neck of the pancreas is gently dissected from the SMV to create a tunnel (Figure 3D). Blunt dissection is performed in a caudal to cranial direction using suction, and small vessel branches draining into the SMV are controlled using LigaSure.
Lymph node dissection around the common hepatic artery and hepatoduodenal ligament
Extent of lymph node (LN) dissection during LPD is similar to that of open surgery. The CHA, proper hepatic artery and portal vein are fully dissected and skeletonized. The LNs and lymphatic tissue at the right site of the celiac axis and SMA are cleared. LNs are removed in an en bloc fashion. First, to better expose the superior border of the pancreas, the liver is retracted by a fan retractor, which is inserted through the epigastric trocar. The lymph nodes are dissected along the superior border of the pancreas to approach the CHA (Figure 4A). When the CHA is identified, dissection is continued in a right to left direction until the coronary vein is exposed. The coronary vein is ligated with endoclips, and lymph nodes lateral to the left gastric and celiac artery are dissected. As further node dissection is performed toward to the right side, the gastroduodenal artery is isolated, doubly ligated with hem-o-lok clips, and divided with LigaSure (Figure 4B). Thereafter, the dissection continues upward along the proper hepatic artery, and the root of the right gastric artery is divided (Figure 4C). A vessel sling is placed around the artery for traction. As the hepatic artery is gently retracted to the left, the left side of the portal vein is dissected. As opposed to arteries which should not be grasped, the portal vein may be grasped with an atraumatic grasper to aid dissection. The coronary vein usually branches from the left side of the portal vein, and should be identified, clipped and divided. The lymph nodes around the common hepatic artery are circumferentially dissected (Figure 4D). For en-bloc resection of lymph nodes, the dissected lymph nodes are placed to the left of the portal vein.
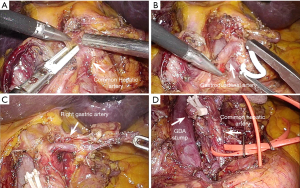
During LN dissection, it is important not to grasp the lymph node itself, because any handling of lymph nodes can cause node rupture and bleeding. It is beneficial to use narrow-edged forceps as the left-hand instrument, since handling and retracting connective tissue around the lymph nodes can be done more precisely (Figure 4A). To avoid development of arterial pseudoaneurysms, lymphatic or nerve tissues on the artery are not removed aggressively.
Division of the bile duct
First, the gallbladder is dissected from the liver bed in an anterograde fashion and the cystic artery is divided. The cystic duct is preserved to achieve en-bloc resection. Then the bile duct is freed from the portal vein and right hepatic artery. A suture is passed around the bile duct, and tied lower to the resection level. After a bulldog clamp is placed on the proximal bile duct, the bile duct is divided with an endoscissor. When necessary, frozen sections are sent for assessment of the bile duct margin. The divided bile duct and dissected lymph nodes are dissected further inferiorly, so the only remaining structures in the hepatoduodenal ligament are divided proximal bile duct, the portal vein and the hepatic artery.
Transection of the pancreas
After creating a retropancreatic tunnel above the SMV, a suture is placed in the inferior aspect of the pancreas, about 1cm left to the resection level. This suture can alleviate bleeding during transection, and can also be used for transection (Figure 5A). With retraction on this suture to the left, transection of the pancreas neck is performed using a ultrasonic shear (Figure 5B). The pancreatic duct is usually located posterior and superiorly within the pancreas parenchyma. As the dissection approaches the location of the pancreatic duct, only the tip of the active jaw is used without closing the jaw, to avoid inadvertent sealing of the pancreatic duct. The pancreatic duct can be identified as a whitish tubular structure, which can usually be distinguished from the adjacent parenchyme. The pancreatic duct is divided using endoscissors to obtain a clear duct margin (Figure 5C). After division, the proximal stump is mobilized from the portal vein. Care should be taken to identify and divide any small branches from the pancreas head. The distal stump is also freed for about 2 cm from the splenic vein, to facilitate the posterior parenchymal sutures of the pancreaticojejunostomy (Figure 5D).
Division of the proximal jejunum
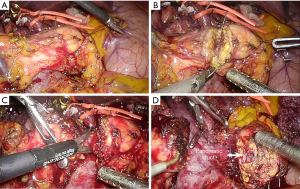
By pulling on the distal end of the duodenum, the proximal jejunum is pulled upward above the colon. When deciding the level of jejunum transection, the redundancy of the afferent loop is important. Regardless of the length of jejunum to be resected, the afferent loop should be redundant enough so a tension-free pancreaticojejunostomy and choledochojejunostomy is possible. After deciding the transection level, countertraction is applied in both sides, and the mesentery is divided (Figure 6A). A linear stapler is inserted to divide the jejunum (Figure 6B). After division, the proximal jejunum is held up by the assistant so the mesenteric side faces the right. The mesentery is divided close to the bowel using the LigaSure, down to the pancreas uncinate process (Figure 6C,D).
Uncinate process dissection
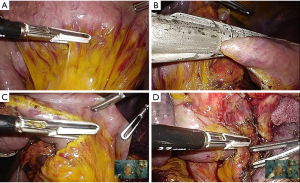
For preparation of the uncinate process mobilization, small branches of the SMV from the pancreas head should be controlled to avoid bleeding during retraction of SMV. When the uncinate process dissection is started, great care is taken not to injure small branches between the first jejunal trunk and pancreas. Since the first jejunal trunk has a highly variable anatomy, dissection around this area should always be performed with caution. After the uncinate is dissected off the first jejunal trunk, the lateral aspect of the SMA is exposed when the SMV is retracted toward the left side (Figure 7A). The mesopancreas is dissected from the SMA in a caudal to cranial direction and the inferior pancreaticoduodenal artery encountered during the dissection is divided (Figure 7B). After further dissection, the previously dissected lymph nodes are pulled down under the portal vein. As dissection continues to the right side of the celiac trunk (Figure 7C), the remaining connective tissue is divided to complete the en-bloc resection (Figure 7D).
Anastomosis
Preparation
The thin area in the mesocolon where the duodenum was previously adhered is divided to create a hole. The jejunal stump is passed through this hole, and brought up to the remnant pancreas. A long enough segment of jejunum should be retracted through the hole to ensure a tension-free anastomosis.
Pancreaticojejunostomy (PJ)
For pancreatic reconstruction, the two-layer, duct-to-mucosa PJ is performed in an end-to-side fashion. The operator stands between both legs of the patient and the assistant standing in the left side of the patient retracts the liver using the epigastric trocar. The camera is inserted through the right lower 12 mm trocar. The operator uses the right upper 5mm trocar as the left-hand port and one of three trocars as the right-hand port, according to the angle of sutures; the left lower 12mm trocar is mainly used, but the left upper 5mm and umbilical 12mm trocars can also be used (Figure 8). When the left lower trocar is used, the angle of the needle holder is perpendicular to the anastomosis line, and the outer layer anastomosis between the pancreas parenchyma and jejunum can be performed. Using one of the three trocars alternately, various angles of suturing are possible, which improve the limited degree of freedom inherent in laparoscopic anastomosis during the duct-to-mucosa anastomosis.
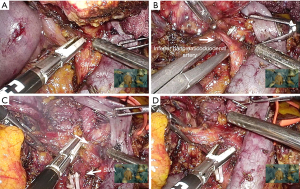
First, a continuous suture is placed between the pancreas parenchyma and jejunum for the posterior row, using a prolene 4-0 suture (Figure 9A). For accurate sutures, it is better to leave all the sutures loose and to tighten at the end, rather than tightening each suture after placement. After completion of the posterior row, another prolene suture for the anterior row is placed and tied to the posterior row suture.
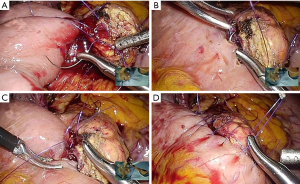
Thereafter, a small hole in the jejunum is made on the opposing side of the pancreatic duct, using electrocautery. Five to eight polydioxanone (PDS) 5-0 sutures are used for a duct-to-mucosa anastomosis depending on the size of the pancreatic duct. Starting with the 12 o’clock suture, the posterior duct-to-mucosa sutures are placed (Figure 9B). Each suture is tied after the next suture is placed. This technique allows each suture to be properly spaced, and also prevents adverse tension to the previous tie while the next suture is placed. After completion of the posterior anastomosis, a pediatric feeding tube with a length of 5–6 cm is placed into the pancreatic duct and the jejunum when the pancreas is soft and the pancreatic duct is not dilated. Then, the anterior duct-to-mucosa anastomosis is performed, by placing two or four sutures between the pancreatic duct and the jejunum (Figure 9C). The sutures are tied after all the sutures are placed, in a cranial to caudal direction. Finally, for the anterior parenchymal suture, continuous running sutures are placed on the pancreas parenchyma and jejunum, in a caudal to cranial direction (Figure 9D). As with the posterior sutures, the sutures are left loose after each suture, and tightened after completing the continuous sutures. After tightening, this suture is tied with the posterior parenchymal suture that was left long to tie later. After completion of the PJ, a polyglycolic acid mesh is placed circumferentially around the anastomosis, and fibrin glue is applied.
Choledochojejunostomy (CJ)
For the CJ, the operator stands to the left side of the patient and the camera is inserted through the umbilical trocar. The epigastric 12 mm-trocar is used as the right hand port, and the right lower 12 mm trocar is used for the left hand port (Figure 10). When the needle holder is inserted through the epigastric trocar, the angle is perpendicular to that of the anastomosis line, and CJ can be performed. The assistant standing on the opposite side of the operator retracts the liver using the right upper trocar. After removing the bulldog clamp placed on the CBD, an opening is made on the jejunum using electrocautery, at a location suitable for a tension free anastomosis.
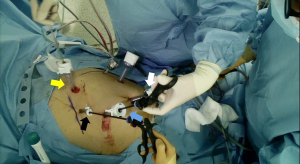
An end-to-side CJ is performed using continuous sutures on the posterior row and interrupted sutures on the anterior row. First, the right edge of the bile duct and the jejunum are sutured and tied (Figure 11A). Then, the posterior row of anastomosis is done in a continuous fashion using a V-loc suture (Figure 11B). The anterior layer is performed with interrupted sutures in a left to right direction, using monosyn 4-0 sutures (Figure 11C). After completion of anastomosis, a dry gauze is compressed on both the anterior and posterior sides of the anastomosis to check for bile leak (Figure 11D).
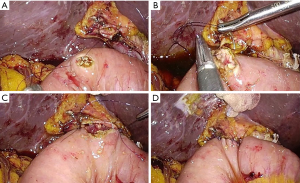
Duodenojejunostomy (DJ)
DJ is performed through the extended umbilical port for removal of the surgical specimen. For preparation of DJ, the jejunum approximately 40 cm distal from the colon mesentery is sutured with the right edge of the duodenal stump. The jejunum immediately distal to the sutured area is marked with ink, to ensure a correct direction of anastomosis during the extracorporeal anastomosis. After the surgical specimen is removed through the 5 cm-sized, extended umbilical wound, the duodenal stump sutured with the jejunum is brought out through this incision, and DJ is performed.
Discussion
Although favorable outcomes of LPD have been reported in many studies, this operative procedure is still performed by a limited number of experts due to a long learning curve, which is related to technical difficulty. Several studies showed that a minimum 30 to 60 cases are required to overcome the learning curve of LPD (8-10). In our experience, surgical failure, which is defined by severe postoperative complications and open conversion, decreased after 60 cases and stabilized about 84 cases (data not shown). Therefore, for wide and safe dissemination of this challenging procedure, efforts should be made to shorten the long learning curve and to minimize unfavorable outcomes in the early period of the learning curve.
Surgeons use various surgical techniques for port placement, resection, and reconstruction according to each individual’s training background, open surgical technique, and accumulated experience. To shorten the learning curve, it is essential to standardize their surgical techniques. For standardization, a long and complex operative procedure needs to be broken down into several steps, as we have divided the resection part into 9 steps. Difficult points in each step should be checked and practiced repeatedly, based on teachings from other experienced surgeons. Documents or videos that describe the procedures performed by experts in detail are useful (11-13). Kuroki et al. introduced various methods of training for LPD, such as video training, 3D simulation, cadaver training and mental skills training (14). A systematic training program is also important for surgeons to achieve satisfactory outcomes with a short learning curve. Recent reports revealed that a well-structured training program under expert guidance at high volume centers reduced the learning curve time of a second-generation surgeon even in low-volume clinical settings (15,16).
The two major postoperative complications that may compromise the safety of patients during the learning curve period are post pancreatectomy hemorrhage (PPH) and postoperative pancreatic fistula (POPF). PPH occurs commonly after PD, and the mortality rates related with PPH are reported to be as high as 12–24% (17,18). Especially late PPH caused by pseudoaneurysm of major arteries is reportedly more dangerous than early PPH, suggesting the necessity of meticulous surgical technique around major arteries. Energy devices, which are frequently used in minimally invasive surgery, have the risk of lateral thermal damage to the arterial wall. Therefore, during lymph node dissection, the energy device should be applied to the loose tissue between the lymph nodes and the artery, and not directly on the artery wall, and lymphatic or nerve tissue should not be removed aggressively. We experienced several cases of PPH caused by arterial pseudoaneurysm in the early period but with increasing experience, we started to take great care not to damage the arterial wall, and this complication decreased. Despite improvements of both surgical technique and perioperative management, POPF remains an unsolved dilemma for surgeons who perform PD. Although a number of studies showed similar POPF rates between open PD and LPD, laparoscopic pancreatic anastomosis is the biggest barrier to surgeons who are planning to perform LPD. Especially the limited range of motion of laparoscopic instruments is difficult to overcome. We presented tips for pancreatic reconstruction to improve the limited degree of freedom. First, the main trocar is located so that the needle is placed perpendicular to the anastomosis line. When the needle holder is introduced through the left lower trocar, which is aligned to the pancreatic resection line, the outer layer suturing between the pancreas parenchyma and the jejunum can be easily made. However, it is difficult to perform the duct-to-mucosa anastomosis requiring various angles of suturing using only one trocar. In our technique, alternative use of other trocars located in the umbilicus and left upper abdomen are beneficial in performing the 360-degree duct-to-mucosa anastomosis. Using this technique, we maintain a standardized suturing method of pancreaticojejunostomy, with minimal variations among different cases.
Conclusions
LPD is a complex operation with a long learning curve. Standardization of the procedure and a dedicated training program is essential to shorten the learning curve and minimize the harm to patient safety especially during the early period of the learning curve.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edoardo Rosso) for the series “Mini-invasive pancreaticoduodenectomy: are we moving from a “feasible” intervention to be considered the standard?” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-52). The series “Mini-invasive pancreaticoduodenectomy: are we moving from a “feasible” intervention to be considered the standard?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. [Crossref] [PubMed]
- Palanivelu C, Jani K, Senthilnathan P, et al. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg 2007;205:222-30. [Crossref] [PubMed]
- Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg 2020;271:54-66. [Crossref] [PubMed]
- Palanivelu C, Senthilnathan P, Sabnis SC, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg 2017;104:1443-50. [Crossref] [PubMed]
- Poves I, Burdio F, Morato O, et al. Comparison of Perioperative Outcomes Between Laparoscopic and Open Approach for Pancreatoduodenectomy: The PADULAP Randomized Controlled Trial. Ann Surg 2018;268:731-9. [Crossref] [PubMed]
- van Hilst J, de Rooij T, Bosscha K, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 2019;4:199-207. [Crossref] [PubMed]
- Lu C, Jin W, Mou YP, et al. Analysis of learning curve for laparoscopic pancreaticoduodenectomy. J Vis Surg 2016;2:145. [Crossref] [PubMed]
- Nagakawa Y, Nakamura Y, Honda G, et al. Learning curve and surgical factors influencing the surgical outcomes during the initial experience with laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2018;25:498-507. [Crossref] [PubMed]
- Wang M, Meng L, Cai Y, et al. Learning Curve for Laparoscopic Pancreaticoduodenectomy: a CUSUM Analysis. J Gastrointest Surg 2016;20:924-35. [Crossref] [PubMed]
- Asbun HJ, Harada E, Stauffer JA. Tips for laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2016;23:E5-9. [Crossref] [PubMed]
- Cho A, Yamamoto H, Kainuma O. Tips of laparoscopic pancreaticoduodenectomy: superior mesenteric artery first approach (with video). J Hepatobiliary Pancreat Sci 2014;21:E19-21. [Crossref] [PubMed]
- Navarro JG, Kang CM. Pitfalls for laparoscopic pancreaticoduodenectomy: Need for a stepwise approach. Ann Gastroenterol Surg 2019;3:254-68. [Crossref] [PubMed]
- Kuroki T, Fujioka H. Training for laparoscopic pancreaticoduodenectomy. Surg Today 2019;49:103-7. [Crossref] [PubMed]
- Chedid AD, Chedid MF, Winkelmann LV, et al. Achieving good perioperative outcomes after pancreaticoduodenectomy in a low-volume setting: a 25-year experience. Int Surg 2015;100:705-11. [Crossref] [PubMed]
- Song KB, Kim SC, Lee W, et al. Laparoscopic pancreaticoduodenectomy for periampullary tumors: lessons learned from 500 consecutive patients in a single center. Surg Endosc 2020;34:1343-52. [Crossref] [PubMed]
- Kasumova GG, Eskander MF, Kent TS, et al. Hemorrhage after pancreaticoduodenectomy: does timing matter? HPB (Oxford) 2016;18:861-9. [Crossref] [PubMed]
- Lu JW, Ding HF, Wu XN, et al. Intra-abdominal hemorrhage following 739 consecutive pancreaticoduodenectomy: Risk factors and treatments. J Gastroenterol Hepatol 2019;34:1100-7. [Crossref] [PubMed]
Cite this article as: Lee JS, Yoon YS, Han HS, Kim J, Lee B, Lee HW, Cho JY. Standard minimally invasive pancreaticoduodenectomy. Laparosc Surg 2021;5:12.