
Robot-assisted pancreaticoduodenectomy with vascular resection: technical details and results from a high-volume center
Introduction
The aggressive biology of pancreatic cancer makes the distinction between curative and palliative treatments largely speculative. However, in the absence of detectable metastases in distant organs, radical resection of the primary tumor is considered the standard of care. Resection of a seemingly localized pancreatic cancer is indeed the only treatment aiming to cure. In patients who will not be eventually cured, resection prolongs survival and improves quality of life when compared to alternative palliative treatments (1,2).
Unfortunately, a truly localized disease permitting resection is observed in only 20% of the patients with pancreatic cancer. The remaining patients are either diagnosed with overtly metastatic disease (50%) or with borderline resectable/locally advanced tumors (30%) (1,3). Borderline resectable tumors (4) are a particular group of neoplasms in which resection is technically possible but at a higher risk of microscopic margin positivity (R1), mostly because of tumor abutment on the superior mesenteric-portal vein. In these patients, typically after neoadjuvant treatments, pancreatoduodenectomy with en-bloc resection of the involved vein segment (PD-VR) is devised to increase the probability of radical resection (R0) (1,5).
Not surprisingly, PD-VR is more complex than pancreatoduodenectomy alone (6-9). Therefore, this operation has been performed mostly through a conventional, open, approach (10,11) In recent years, few specialized centers, have reported small series of PD-VR performed through a minimally invasive approach (10,12-15).
Historically, our group has been proactive in pursuing PD-VR (16). Overall, we have performed over 500 pancreatectomies with associated vascular procedures, including over 150 truly extended procedures including also arterial resections. We have performed the first robot-assisted pancreatoduodenectomy (RAPD) in 2008 and, after some experience, we have started to consider for a robotic approach also patients with limited vascular involvement. We herein report our initial experience with RAPD with en-bloc resection of the superior mesenteric-portal vein [robot-assisted pancreaticoduodenectomy with vascular resection (RAPD-VR)] and we describe the technique that we have developed. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ls-20-94).
Methods
A prospectively maintained database was retrospectively revised to identify all patients who received a RAPD-VR between May 2011 and December 2019 at General Surgery and Transplant Unit (Azienda Ospedaliero Univeristaria Pisana; University of Pisa, Italy). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of University of Pisa (CEAVNO) and informed consent was taken from all the patients.
Selection criteria
In general, as detailed previously (17,18), patients were carefully selected for RAPD. Concerning vascular involvement, at the beginning of our experience all types of vascular involvement was considered an absolute contraindication for a robotic approach. After some experience, limited vein involvement (i.e. unilateral contact, <180°, without distortion of vessel contour) was accepted when patients were considered otherwise suitable for RAPD (15). Overt arterial encasement was considered an absolute contraindication. Limited arterial involvement was considered on a case-by-case basis with great caution.
Outcome measures
The following parameters were recorded: operative time, estimated blood loss, rate of conversion, post-operative complications [classified according to the Dindo-Clavien (19)], incidence and severity of post-operative pancreatic fistula (POPF) (20), incidence and severity of delayed gastric emptying (21), and incidence and severity of post-pancreatectomy hemorrhage (22). Complications graded ≥ III were considered severe. The overall burden of postoperative complications was defined using the comprehensive complication index (23). Post-operative mortality was considered as any death occurring during the first 90 days or during the initial hospital stay if longer than 90 days. Overall survival (OS) and the disease-free survival (DFS) were calculated using the Kaplan-Meier method. The collection of the variables was prospective and through clinics during the first visit and the hospital staying. The follow-up was collected in the clinics or through interview.
Surgical technique
The surgical technique for RAPD-VR and RAPD was previously reported (24). Briefly, resectability was established after an artery first approach to both the superior mesenteric artery (SMA) and the celiac trunk (CT) (Figure 1). The pancreatic head was fully mobilized en-bloc with the lympho-neural tissue lying between the SMA and the CT (so called mesopancreas). No attempt was made to detach the vascular segment from the tumor, with the aim of maximize the probability to achieve an R0 resection (25,26) (Figure 2) (Video 1). In general, segmental vein resection was preferred over tangential resection. In case of segmental vein resection, depending on the length of the resected vessel, reconstruction was performed either by direct end-to-end anastomosis (type III vein resection) (Video 2) or using an interposition graft (type IV vein resection) (Video 3) (4). In case of a large side-wall resection, vein repair is performed using a large peritoneal patch or using an autologous vein patch (Video 1) (14). When resection involved the spleno-mesenteric junction, splenic vein drainage was always restored. The splenic vein was either reimplanted on the reconstructed porto-mesenteric vein or on the inferior vena (Figure 3A,B).
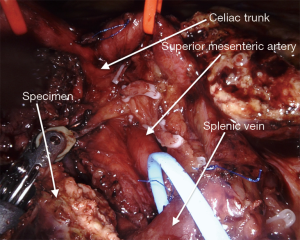
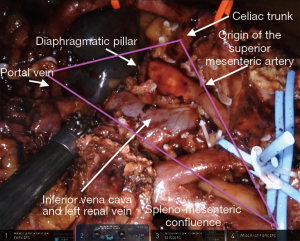
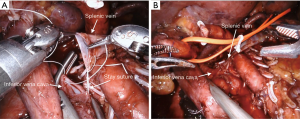
Vascular sutures were performed using fine sutures (6/0 or 7/0) of expanded polytetrafluoroethylene (Gore, W. L. Gore & Associates, USA).
Histopathological assessment
Pathology analysis of resected specimens was conducted according to the Leeds protocol (15,27). Briefly, circumferential margins were identified and stained using different colors in the fresh specimen. After fixation in 10% buffered formalin the specimen was sliced in <5-mm-thick axial slices. Each slice was examined in a single large slide. Seven margins were assessed: anterior surface, posterior surface, vein bed, SMA groove, pancreatic neck, proximal duodenum/stomach, and common bile duct. Margins were defined positive (R1) if cancer cells were identified ≤1 mm of any margin.
Statistical analysis
Fisher’s exact test and Pearson Chi square test were used to compare categorical variables between groups as appropriate. The relation between the independent variable “length of the resected vein” and the dependent variable “need for jump graft” was evaluated with a logistic regression. The cut off-value of the independent variable was calculated with receiver operating characteristic (ROC) curves.
ROC curves were used to calculate the cut-off value for using a vascular graft for vein reconstruction.
OS and DFS were calculated by using Kaplan-Meier curves.
Statistical analysis was carried out using JMP® 9.0.1 software package for Mac, Copyright© SAS Institute Inc., SAS campus Drive, Cary, NC, USA.
Results
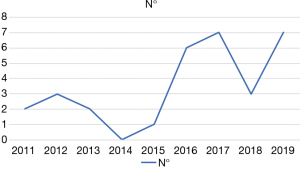
During the study period a total of 184 RAPDs were performed. RAPD-VR was performed in 26 patients (14.1%). As shown in Figure 4, the number of RAPD-VR significantly increased during the last 4 years (P=0.01; Chi square). The results of 22 patients (84.6%) who received an isolated vein resection are presented herein. The remaining 4 patients received either an isolated arterial resection (n=3; 11.5%) or a combined arterial and venous resection (n=1; 3.8%).
The baseline characteristics of the study population are summarized in the Table 1. Vein involvement was suspected based on imaging findings in 14 patients (63.6%). In these patients RAPD-VR was planned before surgery. In the remaining 8 patients tumor adherence to the porto-mesenteric vein was discovered during the procedure. The decision to proceed with robotic resection, instead of converting the patients to an open approach, was based on the limited extension of vascular involvement (that was instead missed at preoperative studies) and the judgement that resection could have been performed safely even when associating a vascular procedure.
Table 1
| Characteristics | Value |
|---|---|
| Number of patients (%) | 22 (100.0) |
| Age, years, mean (±SD) | 63 (±9.5) |
| Gender, males, n (%) | 9 (40.9) |
| Body mass index, kg/m2, mean (±SD) | 24.1 (±2.9) |
| Prior abdominal surgery, n (%) | 16 (72.7) |
| ASA score, median [IQR] | 2 [2–3] |
| Age-adjusted CCI index, median (±SD) | 3.3 (±1.4) |
| Neoadjuvant chemotherapy, n (%) | 1 (4.5) |
| Heart disease, n (%) | 2 (9.1) |
| Hypertension, n (%) | 9 (40.9) |
| Diabetes, n (%) | 4 (18.2) |
| Symptoms, n (%) | 18 (81.8) |
| Pain, n (%) | 12 (54.5) |
| Jaundice, n (%) | 11 (50.0) |
| Weight loss, n (%) | 5 (22.7) |
| Percutaneous biliary drainage, n (%) | 1 (4.5) |
| Endoscopic biliary stenting, n (%) | 5 (22.7) |
| CA19-9, median [IQR] | 43 [15–137] |
ASA, American Society of Anesthesiologists; Ca, carcinoma; CCI, Charlson Comorbidity Index; SD, standard deviation; IQR, interquartile range.
The main operative and post-operative results are summarized in Table 2. There was one conversion to open surgery (1/22; 4.5%). This conversion occurred at the end of the procedure because of diffuse bleeding in a patient with previous bone marrow transplant and multiple comorbidities. The pylorus was spared in nearly all patients (19/22: 86.4%), as per our institutional policy (28). The mean vascular clamping time was 33.75±9.2 minutes and the mean vascular anastomotic time was 20.5±2.6 minutes. Despite long operative times (610.0±83.5 minutes) only 2 patients (9.1%) required intraoperative blood transfusions. The superior mesenteric vein was resected in 9 patients (40.9%), the portal vein in 3 patients (13.6%) and the porto-mesenteric junction in 10 patients (45.5%). There were 1 type I (4.5%), 3 type II (13.6%), 10 type III (45.5%) and 8 type IV (36.4%) resections. In type II resection the side-wall defect was closed using a peritoneal patch graft in two patients (29) and a patch of right gonadal vein in one patient. In type IV resections the left internal jugular vein was used as jump graft in 7 patients. In one patient the interposition graft was a paneled saphenous vein graft. Need to use of a jump was associated with the length of resected vein segment (P=0.04; Chi square), with a cut-off of 1.3 cm (AUC =0.78).
Table 2
| Variables | Value |
|---|---|
| Operative time, minutes, mean (±SD) | 610.0 (±83.5) |
| Estimated blood loss, mL, median (IQR) | 889.7 (719.4–1,430.2) |
| Patients receiving intraoperative blood transfusions, n (%) | 2 (9.1) |
| Pylorus preservation, n (%) | 19 (86.4) |
| Postoperative complications, n (%) | 14 (63.6) |
| Grade I | 1 (4.5) |
| Grade II | 8 (36.4) |
| Grade IIIa | 1 (4.5) |
| Grade IIIb | 2 (9.1) |
| Grade VIa | 0 |
| Grade VIb | 0 |
| Grade V | 2 (9.1) |
| Severe postoperative complications (≥ grade III), n (%) | 5 (22.7) |
| Comprehensive complication index, median [IQR] | 29.6 [0–31] |
| Post-operative pancreatic fistula, n (%) | 3 (13.6) |
| Grade BL | 2 (9.1) |
| Grade B | 1 (4.5) |
| Grade C | 0 |
| Delayed gastric emptying, n (%) | 7 (31.8) |
| Grade A | 0 |
| Grade B | 4 (18.2) |
| Grade C | 3 (13.6) |
| Post-pancreatectomy hemorrhage, n (%) | 2 (9.1) |
| Grade A | 0 |
| Grade B | 0 |
| Grade C | 2 (9.1) |
| Length of hospital stay, days, median [IQR] | 15 [12–28] |
| Reoperation, n (%) | 4 (18.2) |
| 90-day readmission, n (%) | 4 (18.2) |
| Patency of the vascular reconstructions, n (%) | 19 (86.4) |
SD, standard deviation; IQR, interquartile range.
The pancreatic remnant was managed by means of duct-to mucosa anastomosis in all procedures. In the first 10 consecutive patients a Cattell-Warren pancreaticojejunostomy was performed (45.5%) while in the following 12 patients a modified Blumgart pancreaticojejunostomy was adopted (54.5%). Using these techniques, one patient developed a grade B POPF (4.5%), and two patients a biochemical leak.
Two patients died after RAPD-VR. Both deaths occurred early on during this experience. In the overall series of RAPD these fatalities occurred at case 23 and 46, respectively, in the series of RAPD-VR at case 1 and 5, respectively.
Patency of vein reconstruction was documented in 19 patients (86.4%). One asymptomatic patient developed vein thrombosis that was incidentally discovered during the initial hospital stay. The patient was treated with intravenous infusion of sodium heparin and the patient had no clinical consequences.
Final pathology diagnosis was pancreatic ductal adenocarcinoma in 18 patients (81.8%). Other tumor types were adenosquamous carcinoma (n=2; 9.1%), malignant intraductal papillary mucinous neoplasm (n=1; 4.5%), and neuroendocrine cancer (n=1; 4.5%). R1 resection was documented in 11 patients (50.0%). Six had R1 at multiple margins (27.3%). Vein infiltration was confirmed in 13 specimens (59.1%). A summary of histopathology analysis of resected specimens is provided in Table 3.
Table 3
| Variables | Value |
|---|---|
| T stage, n (%) | |
| T1 | 0 (0) |
| T2 | 8 (36.4) |
| T3 | 14 (63.6) |
| T4 | 0 (0) |
| N stage, n (%) | |
| N0 | 4 (18.2) |
| N1 | 14 (63.6) |
| N2 | 4 (18.2) |
| R1, n (%) | 11 (50.0) |
| Tumor size, mm, median (IQR) | 3.0 (2.5–3.7) |
| Number of examined lymph nodes, mean (±SD) | 42.2 (±16.3) |
| Lymph node ratio, median (IQR) | 0.08 (0.006–0.2) |
| Number of positive lymph nodes, n (%) | |
| 0 | 4 (18.2) |
| 1–3 | 10 (45.5) |
| 4–6 | 3 (13.6) |
| ≥7 | 5 (22.7) |
| Confirmed vascular infiltration, n (%) | 13 (59.1) |
| Depth of vascular infiltration, n (%) | |
| Intima | 4 (30.8) |
| Media | 6 (46.2) |
| Adventitia | 3 (23.1) |
| Length of venous infiltration, mm, median [IQR] | 8 [5–20] |
SD, standard deviation; IQR, interquartile range.
After a median follow-up period of 19.9 (5.0–33.3) months, median OS was 39.7 (27.5–not available) months. Tumor recurrence was documented in 6 patients (27.3%). Isolated local recurrence developed in 1 patient (4.5%). Mean DFS was 32.9 months (11.5–45.8).
Discussion
In open surgery we have pursued PD-VR since the late 1980s (16). Overall, we have performed over 500 pancreatectomies with associated vascular procedures and we have established standardized techniques to face all operative scenarios. In essence, our efforts were finalized not just to develop methods permitting safe handling and reconstruction of large peripancreatic vessels, but also oncologically sound procedures aimed at maximizing local radicality. In right-sided resections, these techniques include an artery first approach to both SMA and CT, with en-bloc removal of the lympho-neural tissue lying in the triangle between the superior mesenteric-portal vein, the SMA, and the CT (so called mesopancreas or extrapancreatic neural plexus). No attempt is made to detach the tumor from the vein or to thin down tumor adherence so that eventually a side-wall, limited, resection could be performed. As a consequence, most of our patients receive a segmental vein resection with either type III or type IV reconstruction. We rarely perform type I resections, unless tumor adherence to the vein is extremely limited. When a side-wall resection of the vein is performed, this is typically a generous resection mandating for patch repair (type II resection).
In this study, even if with the biases connected to a monocentric retrospective analysis and the small size of the cohort, we have presented the results of 22 RAPD-VR. In keeping with the principles that we have established in open surgery, 18 of 22 RAPD-VR (81.8%) were segmental vein resections. The other four procedures were type I resection in one patient and type II resection in three patients. Therefore, robotic assistance permits to faithfully reproduce the open technique. However, our results are unique. Indeed, in the largest series of RAPD-VR reported so far 50 procedures are described including 43 type I resections (27 managed by linear stapler and 16 by venorrhaphy), 6 type II resections, and I type III resection (12). These figures show that different approaches to vein resection and reconstruction are followed at different institutions even when robotic assistance is used. Clearly type I resection adds little to standard RAPD while type IV resection adds, at least, technical complexity. Our series shows that robotic assistance permits all types of resections and reconstructions, in the setting of patients selected for a minimally invasive approach.
Our study also confirms the general feasibility of RAPD-VR, as witnessed by the low conversion rate and the low proportion of patients requiring blood transfusions. Actually, we never had to convert a patient due to difficulties in dissection and/or vein reconstruction. The only case of conversion to open surgery, occurred after completion of tumor resection and vascular reconstruction due to diffuse bleeding, in a patient with previous bone marrow transplant, that was difficult to control under minimally invasive conditions. Our conversion rate favorably compares with the other few series reported in the literature that show a conversion rate between 10% (12) and 36.4% (30). It is important to underscore that our low conversion rate was achieved in the context of low blood transfusion requirements showing that our procedures had a smooth intraoperative course. On the other hand, RAPD-VR appears to be a complex procedure, as demonstrated by long operative times and high mortality rates. Therefore, RAPD-VR should be implemented with caution in centers that have already surpassed their learning curve with the standard procedure (31,32). We also believe that background experience in PD-VR is important. A critical appraisal on mortality following RAPD-VR shows that we do not have enough data to draw final conclusions on this regard. Indeed, we have reported a mortality rate of 9%, caused by two deaths occurring in 22 procedures. Beane and coworkers reported a rate of 8% in 50 procedures (12), while Shyr and coworkers described no deaths in 11 patients (30). Other, small, series simply did not report on post-operative mortality (33). The yet limited number of procedures could overemphasize the relative impact of rare events, such as post-operative mortality making current rates still immature for critical evaluation. However, RAPD-VR has a mortality risk that should not be underestimated. Concerning the present series, we had two post-operative deaths. The clinical history of the first of these two patients begins with a bleeding episode originating from a pancreaticoduodenal artery. Hemorrhage was fixed at repeat surgery but the patient eventually died 40 days after the index operation due to widespread pneumonia with multi-resistant bacteria. The second patient suddenly bled on postoperative day fifteenth due to a “tear” close to the origin of the SMA, in the absence of signs of POPF and/or local sepsis. The breach was promptly repaired without massive blood loss but the patient developed intraoperative cardiac arrest and could not be resuscitated. We speculated that this vascular lesion was caused by unintentional thermal injury during arterial dissection. This one of the main reasons why we do not employ energy devices close to major arteries. We prefer to perform these dissections using cold scissors, and seal lymphatic channels and vessels using a combination of clips and ligatures (24). This technique can be tedious and is time consuming, but we have not observed other cases of this type of postoperative bleeding in 168 consecutive RAPDs.
We have already underscored that in RAPD-VR we tried to duplicate the technique that we have established in the open procedure. Indeed, we performed 18 segmental vein resections. In open surgery, after a Cattel-Braasch maneuver, the use of a jump graft is rarely required even when the length of the resected vein segment exceeds three centimeters. In minimally invasive approach, the fact that the Cattel-Braasch maneuver is unpractical and that the reverse Trendelenburg position further outdistance the two cut ends of the vein, a jump graft could be required more frequently. We have shown that direct reconstruction is still feasible in over half of the patients undergoing segmental vein resection (11/19; 57.9%). An interposition graft is required when the length of the resected vein segment exceeds 1.3 cm.
The rate of R0 resection is an important quality metric in pancreatic oncologic surgery. In our series we observed a rate of R1 resections of 52.2%. Is important to underscore that this result was observed after systematic study of specimens according to stringent pathology methods and in a series of patients who did not receive neoadjuvant treatments. The use of these treatments, also in the setting of RAPD, was shown to increase the rate of R0 resections (12). The oncologic adequacy of our technique is shown also by the number of retrieved lymph nodes that largely exceeds the standard defined by the American Joint Committee on Cancer for staging of pancreatic cancer (34). The fact that only one patient developed isolated local recurrence further reinforces the value of our approach.
It is also important to underscore that we have observed only one grade B POPF giving an overall rate of clinically relevant POPF of approximately 4%. This result is in keeping with previous observations (12), and is probably related to the fact that nearly all patients requiring RAPD-VR are affected by pancreatic cancer so that they have firm gland texture and enlarged main pancreatic ducts.
Prevention of vein thrombosis after PD-VR and RAPD-VR is important. Our general policy, however, does not encourage enhanced anticoagulation after vein resection and reconstruction (16). One vein thrombosis was observed in the early postoperative course, without clinical consequences. Our rate of thrombosis is in keeping with data reported in the literature (35) and suggests that there is probably no need for a specific antithrombotic protocol in these patients (36).
In conclusion, RAPD-VR is feasible, but safety remains to be established. As more data are needed to draw final conclusions on safety of RAPD-VR, we discourage groups with limited experience in PD-VR and/or that have not surpassed the learning curve in RAPD to embark upon these extra complex procedures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edoardo Rosso) for the series “Mini-invasive pancreaticoduodenectomy: are we moving from a “feasible” intervention to be considered the standard?” published in Laparoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ls-20-94
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ls-20-94
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-94). The series “Mini-invasive pancreaticoduodenectomy: are we moving from a “feasible” intervention to be considered the standard?” was commissioned by the editorial office without any sponsorship or funding. UB serves as an unpaid editorial board member of Laparoscopic Surgery from Nov 2017 to Nov 2021. The authors have no other conflict of interest and nothing to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the University of Pisa (CEAVNO) and informed consent was taken from all the patients
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699-708. [Crossref] [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with Major Vascular Resection: a Comparison of Laparoscopic Versus Open Approaches. J Gastrointest Surg 2015;19:189-94. [Crossref] [PubMed]
- Ravikumar R, Sabin C, Hilal MA, et al. Portal vein resection in borderline resectable pancreatic cancer: A United Kingdom multicenter study. J Am Coll Surg 2014;218:401-11. [Crossref] [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [Crossref] [PubMed]
- Kaneoka Y, Yamaguchi A, Isogai M. Portal or superior mesenteric vein resection for pancreatic head adenocarcinoma: Prognostic value of the length of venous resection. Surgery 2009;145:417-25. [Crossref] [PubMed]
- Al-Haddad M, Martin JK, Nguyen J, et al. Vascular resection and reconstruction for pancreatic malignancy: A single center survival study. J Gastrointest Surg 2007;11:1168-74. [Crossref] [PubMed]
- Siriwardana HPP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg 2006;93:662-73. [Crossref] [PubMed]
- Allema JH, Reinders ME, Van Gulik TM, et al. Portal vein resection in patients undergoing pancreatoduodenectomy for carcinoma of the pancreatic head. Br J Surg 1994;81:1642-6. [Crossref] [PubMed]
- Sindelar WF. Clinical Experience with Regional Pancreatectomy for Adenocarcinoma of the Pancreas. Arch Surg 1989;124:127-32. [Crossref] [PubMed]
- Zureikat AH, Postlewait LM, Liu Y, et al. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg 2016;264:640-9. [Crossref] [PubMed]
- King JC, Zeh HJ, Zureikat AH, et al. Safety in Numbers: Progressive Implementation of a Robotics Program in an Academic Surgical Oncology Practice. Surg Innov 2016;23:407-14. [Crossref] [PubMed]
- Beane JD, Zenati M, Hamad A, et al. Robotic pancreatoduodenectomy with vascular resection: Outcomes and learning curve. Surgery 2019;166:8-14. [Crossref] [PubMed]
- Wang X, Cai Y, Zhao W, et al. Laparoscopic pancreatoduodenectomy combined with portal-superior mesenteric vein resection and reconstruction with interposition graft: Case series. Medicine (Baltimore) 2019;98:e14204 [Crossref] [PubMed]
- Dokmak S, Aussilhou B, Calmels M, et al. Laparoscopic pancreaticoduodenectomy with reconstruction of the mesentericoportal vein with the parietal peritoneum and the falciform ligament. Surg Endosc 2018;32:3256-61. [Crossref] [PubMed]
- Kauffmann EF, Napoli N, Menonna F, et al. Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg 2016;401:1111-22. [Crossref] [PubMed]
- Boggi U, Del Chiaro M, Croce C, et al. Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery 2009;146:869-81. [Crossref] [PubMed]
- Napoli N, Kauffmann EF, Perrone VG, et al. The learning curve in robotic distal pancreatectomy. Updates Surg 2015;67:257-64. [Crossref] [PubMed]
- Napoli N, Kauffmann EF, Palmeri M, et al. The Learning Curve in Robotic Pancreaticoduodenectomy. Dig Surg 2016;33:299-307. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH)-An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7. [Crossref] [PubMed]
- Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. [Crossref] [PubMed]
- Inoue Y, Saiura A, Yoshioka R, et al. Pancreatoduodenectomy with systematic mesopancreas dissection using a supracolic anterior artery-first approach. Ann Surg 2015;262:1092-101. [Crossref] [PubMed]
- Chowdappa R, Challa VR. Mesopancreas in Pancreatic Cancer: Where do We Stand – Review of Literature. Indian J Surg Oncol 2015;6:69-74. [Crossref] [PubMed]
- Verbeke CS, Leitch D, Menon K V, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232-7. [Crossref] [PubMed]
- Mosca F, Giulianotti PC, Balestracci T, et al. Preservation of the pylorus in duodenocephalopancreatectomy in pancreatic and periampullary carcinoma. Chir Ital 1994;46:59-67. [PubMed]
- Dokmak S, Aussilhou B, Sauvanet A, et al. Parietal peritoneum as an autologous substitute for venous reconstruction in hepatopancreatobiliary surgery. Ann Surg 2015;262:366-71. [Crossref] [PubMed]
- Shyr BU, Chen SC, Shyr YM, Wang SE. Surgical, survival, and oncological outcomes after vascular resection in robotic and open pancreaticoduodenectomy. Surg Endosc 2020;34:377-83. [Crossref] [PubMed]
- Shi Y, Wang W, Qiu W, et al. Learning Curve From 450 Cases of Robot-Assisted Pancreaticoduocectomy in a High-Volume Pancreatic Center. Ann Surg 2019; Epub ahead of print. [Crossref] [PubMed]
- Zhang T, Zhao ZM, Gao YX, et al. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc 2019;33:2927-33. [Crossref] [PubMed]
- Marino MV, Latteri MA, Ahmad A. Tangential venous resections during robotic-assisted pancreaticoduodenectomy: the results of a case series (with Video). J Gastrointest Surg 2020;24:1920-1. [Crossref] [PubMed]
- Kamarajah SK, Burns WR, Frankel TL, et al. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol 2017;24:2023-30.
- Snyder RA, Prakash LR, Nogueras-Gonzalez GM, et al. Vein resection during pancreaticoduodenectomy for pancreatic adenocarcinoma: Patency rates and outcomes associated with thrombosis. J Surg Oncol 2018;117:1648-54. [Crossref] [PubMed]
- Ono Y, Tanaka M, Matsueda K, et al. Techniques for splenic vein reconstruction after pancreaticoduodenectomy with portal vein resection for pancreatic cancer. HPB (Oxford) 2019;21:1288-94. [Crossref] [PubMed]
Cite this article as: Kauffmann EF, Napoli N, Menonna F, Genovese V, Cacace C, Andrea CI, Daniela C, Vistoli F, Amorese G, Boggi U. Robot-assisted pancreaticoduodenectomy with vascular resection: technical details and results from a high-volume center. Laparosc Surg 2020;4:37.


