
New technology minimally invasive for the treatment of gastro-esophageal reflux disease: LINX
Inroduction
The clinical syndrome called gastroesophageal reflux disease (GERD) is caused by the reflux of stomach content into the esophagus or also oral cavity and it develops several annoying symptoms and complications (1). GERD negatively affects patients’ quality of life and it is associated with an increased work absenteeism, a low score in sleep scale and a decrease in productivity and physical functioning (2). The prevalence of the disease is higher in western countries compared to Asia: it has been estimated at 20–30% in USA and less than 10% in Japan (3-5). The heartburn is a retrosternal burning feeling, while regurgitation is the perception of refluxed gastric contents into the mouth. They are symptoms sufficiently descriptive to be diagnostic for GERD. Endoscopy can further classify GERD in erosive reflux disease (ERD) and non-erosive reflux disease (NERD).
The primary aim of the GERD therapy is to control symptoms and increase the patients’ Quality of Life (QOL). Furthermore, long-term control of the disease prevents the complications: esophageal stenosis, Barrett’s esophagus and adenocarcinoma (6-9).
Medical treatments are the mainstream for therapy and Proton Pump Inhibitors (PPI) are the first line drugs. Despite the good results with an oral PPI therapy for 8 weeks, several patients experience the PPI-resistant GERD, a condition in which reflux symptoms caused by GERD are not adequately mitigated or esophageal mucosa break did not heal after medical therapies (10). Another possibility for GERD treatment is surgery. Anti-reflux surgery is indicated with moderate level of evidence in PPI-resistant GERD patients and with low level of evidence in erosive GERD patients on long term PPI treatment (10). In western countries several clinical trials have been done to compare surgery and medical treatment and they show an improvement of QOL for at least 1 year, reduction of gastric content reflux and a low rate of Barrett’s esophagus in patients treated with surgery (11-14).
Currently laparoscopic Nissen fundoplication (LNF) is the gold-standard surgical treatment for GERD.
Unfortunately, laparoscopic fundoplication is influenced by surgical experience and skill and the achievement of a secure anti-reflux effect must be balanced with the onset of complications such as dysphagia and bloating (15,16). LINX Reflux Management System (Torax Medical, Maple Grove, MN) is a device for magnetic sphincter augmentation (MSA) appeared in 2008 (17) and approved by FDA in 2012. It has emerged as a valuable alternative surgical approach in patients with GERD (18) instead of LNF.
The aim of this study is to elucidate the indication, surgical implantation and provide a brief review of current literature regarding LINX system and its comparison with the gold-standard surgical technique: LNF.
Device features
The LINX SYSTEM is an alternative way to surgical fundoplication. It’s implantable through a minimal invasive and reversible procedure. During the operation there is no need of an extensive dissection so the anatomical disruption is minimal and the fundic area is spared. The sizing of the device is customizable and adaptable to a wide range of esophagus diameter.
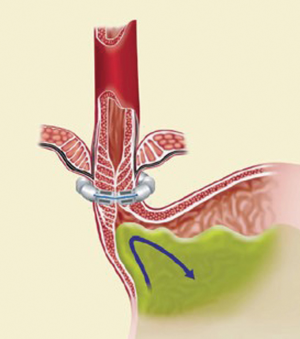
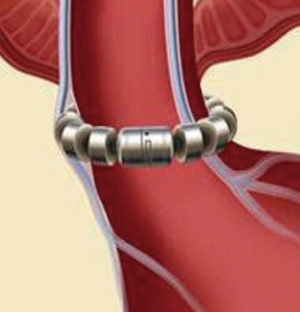
The LINX SYSTEM is manufactured with titanium beads that contain magnetic cores and are linked together using a titanium wire forming a flexible circular ring (Figure 1). This device, after the implantation, rests around the lower esophageal sphincter (LES) (Figure 2). The magnetic cores of the beads are conceived to increase the sphincter’s resistance to opening from gastric pressures (20).
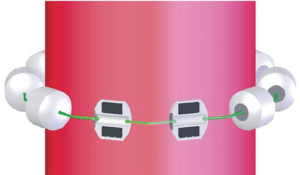
The augmentative force of the device doesn’t decrease over time. When implanted, at rest, there is no compression of the esophageal wall and all the beads are touching each other. During swallowing a bolus can freely pass because the transport force allows the beads to separate and breaks temporarily the attraction bond between magnetic beads because of separation distance. When all beads are separated, the diameter of LINX is almost double. The magnetic attraction force that must be overcome to allow separation of the beads is the same regardless of the number of beads (21).
The LINX SYSTEM is available in various lengths, with different number of beads (from 10 to 18 beads). The device is sized for each patient based on the measurement of the circumference of the esophagus at the gastroesophageal junction (GEJ) (19).
Technical features
Preoperative evaluation
The Gastroesophageal Reflux Disease-Health Related Quality of Life (GERD-HRQL) questionnaire is still the best preoperative evaluation in order to assess the impact of GERD on patients’ everyday life. It is administered off PPI therapy, prior to any diagnostic test (22).
Through esophagogastroduodenoscopy we can verify the presence of esophagitis (Los Angeles classification) and, if present, the length of hiatal hernia (HH; measuring it between the proximal limit of the gastric folds and the crural impression).
LES pressure and length can be tested using esophageal manometry with a station pull-through method: five wet swallows are needed to measure LES relaxation and ten wet swallows for esophageal contractility (5 mL for each swallow, every 30 seconds). The esophageal motility is abnormal when the average contraction amplitude is 30 mmHg or less and/or when there are at least 30% of simultaneous, interrupted or dropped waves.
Intraoperative
The intraoperative conduction is well described in a previous paper by Bonavina et al. (18). Under general anesthesia, the device is implanted laparoscopically.
The exposure of anterior esophageal wall is possible by the dissection of the visceral peritoneum localized on the anterior surface of GEJ. After this, the anterior vagal trunk is detected and preserved in its intramuscular location. The retro-esophageal dissection is performed starting from the anterior border of the right crus, just cephalad to decussation of the crura. In this phase we can identify the posterior vagal trunk. Then the dissection takes place also on diaphragm’s left crus. The procedure goes on with the retro-esophageal opening and with the creation of a tunnel between the posterior vagal trunk and the posterior esophageal wall and then with the placement of a 6 mm Penrose drain encircling the esophagus. Since there are different sizes of LINX, we encircle the esophagus with the measurement tool to select the device suitable for the patient and then the LINX is placed into the tunnel and so it encircles the esophagus (Figure 3). At the end LINX lies in the incision made previously in the visceral peritoneum at the level of GEJ (Figure 4). Both ends of the LINX are securely sutured with a Ti-Knot ® (LSI Solutions, Victor, NY, USA). Endoscopically the location of the implant is the Z line (Figure 5).
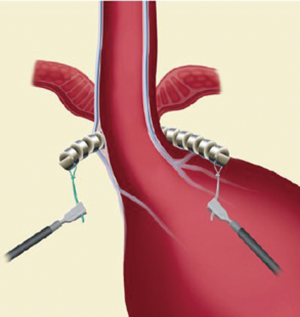
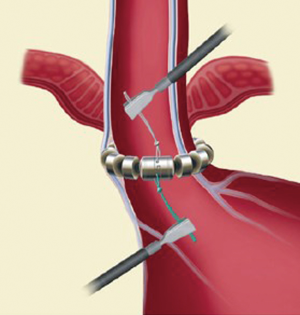
Postoperative period
Oral refeeding starts on postoperative day 1 and the discharge takes place in postoperative day 1 or 2. The follow up is strongly recommended according to the center’s protocols.
Indications for use
The LINX Reflux Management System is used to minimize or eliminate GERD related symptoms despite maximum medical therapy.
Pathologic GERD is defined by abnormal Ph testing.
Contraindication
Allergies to titanium, nickel, ferrous or stainless steel material.
Precautions
- LINX device is labeled for use by physician only.
- For single use only. Do not sterilize.
- The LINX device has not been evaluated in case of HH >3 cm. The use of the device in this subgroup of patients depends on medical history and severity of symptoms.
- Safety and effectiveness have not been evaluated for other several conditions:
- Barrett’s esophagus grade B and C (LA Classification), grade IV (Savary-Miller) esophagitis.
- Patients with defibrillator, pacemaker or other metallic abdominal implants.
- Patients with major motility disorders, stricture or anatomic abnormalities (Schatzki’s ring).
- Patients with scleroderma.
- Patients with suspected or confirmed esophageal or gastric cancer.
- Distal esophageal motility less than 35 mmHg peristaltic amplitude or <70% propulsive peristaltic waves.
- achalasia, nutcracker esophagus, diffuse esophageal spasm or hypertensive LES.
- Prior gastric or esophageal surgery.
- Variceal disease.
- Morbid obesity.
- Lactating, pregnant or plan to become pregnant (21).
Conclusions
GERD is a common disorder worldwide (10–30% of adults); lifestyle modifications and PPI therapy work in many patients with GERD but in 30–40% of them symptoms persist. Patients can become candidate for surgery because of partial control of symptoms with medication, non-compliance with oral treatment, request to avoid long-term PPI therapy, complications or side effects related to PPIs, cost of medical therapy, symptoms with large HH (23).
Currently LNF is the gold-standard surgical treatment for GERD. Magnetic sphincter augmentation device of the LES (MSA or LINX® Reflux Management System, Torax Medical) is an upcoming alternative technique described in 2008 and approved by FDA in 2012.
At present, analysis of literature shows two reviews [Zhang et al. (24) and Schizas et al. (21)] and three meta-analyses [Aiolfi et al. (25), Guidozzi et al. (26) and Chen et al. (27)] comparing LNF and MSA.
Zhang et al. (2016) analyzed the data from 15 clinical studies describing the status of MSA or LINX: they concluded that MSA is as effective as the conventional surgical treatment and there are some advantages with MSA such as good control of symptoms, minimal invasion and less severe postoperative complications.
Chen et al. (2017) included 4 trials comparing MSA and NF with a total of 624 patients (299 MSA vs. 325 NF respectively). This meta-analysis confirmed efficacy and safety of both techniques; it also showed that MSA has shorter operative time, shorter length of stay and fewer complications of gas and bloating than NF.
In the metanalysis of Aiolfi et al. (2018) 7 studies have been included with 1,211 total patients (686 MSA vs. 525 Nissen or Toupet laparoscopic fundoplication). Also, this study demonstrated that both techniques are safe and effective. Moreover, MSA is a less invasive and more standardized procedure and seems to induce less bloating and flatulence and to facilitate belch and vomiting.
Guidozzi et al. (2019) identified 6 studies that compared MSA vs. fundoplication, with a total of 1,099 patients (632 vs. 467 respectively). This systematic review also included 13 single-arm cohort studies (11,598 total patients) which evaluated clinical outcomes using MSA. This analysis also confirmed that MSA is as effective as fundoplication in the control of GERD’s symptoms and suggested MSA may be superior in reduction of gas bloating and improvement of belching. Guidozzi et al. expressed the strong need for a randomized clinical trial between these two surgical treatments.
At last, Schizas et al. (2020) reviewed 35 studies with 2,511 MSA patients. This paper showed the advantages of MSA: this technique has shorter operative time and it can be performed with less technical variability than LNF. Moreover, during MSA less interventions on normal anatomy are needed and after this procedure the patient has fewer bloating symptoms and a better capacity to belch or vomit.
Therefore, all meta-analyses and reviews confirmed safety and efficacy, considered as cessation of PPI therapy and reduction or elimination of symptoms, of both methods. The rate of complications is similar in the two groups: postoperative morbidity is 0–3% in MSA and 0–7% in LF. The major complication is dysphagia which can be solved with an endoscopic dilation. Both techniques improve the quality of life of patients with reduction of bloating symptoms and improvement of belching ability.
If necessary, LINX device can be removed. The main cause that lead to device removal is recurrence of heartburn or regurgitation, not related to device complications; never this procedure was performed emergently. Indeed, literature confirms the safety of LINX device and MSA technique (21).
Lipham et al. (28) in their study showed that only 3.4% of patients were re-operated.
In another study incidence of device removal was 2.7% without complications (29).
Furthermore, in some cases during the procedure the surgeon performed fundoplication, mostly partial, without long-term complications (30); however, there aren’t evidences or studies regarding how to proceed after device removal.
BMI >35 kg/m2 influences negatively the success of MSA (31) and LINX implantation seems promising in patients who underwent bariatric surgery only after losing weight (32,33). Further studies including this subgroup of patients are needed.
LINX device, according to SAGES technology and value assessment committee (34), can be successfully used in patients with HH <3 cm. The instructions for use (IFU) reports HH >3 cm within the precaution. Despite this, recent studies (35,36) demonstrated promising results in using MSA in patients with HH (HH) >3 cm.
Ayazi et al. (37) demonstrated that excellent outcomes after MSA don’t depend on the presence or size of HH and that, despite higher rates of recurrence in large HH than in small ones, the rates of postoperative intervention and LINX removal are similar.
In the meta-analyses and reviews previously described, the presence of HH larger than 3 cm was often an exclusion criterion therefore other studies regarding this population of patients are necessary.
Compared to LNF, MSA has a shorter operative time and length of stay that can neutralize the initial higher cost of the device (38). A study published in 2019 (39) demonstrated that payer costs may be compensated by the reduction in the expenses after surgery.
Future researches are needed in order to present long-term outcomes and confirm efficacy and safety profile, in particular in literature there isn’t a randomized controlled trial of MSA vs. LNF and many authors have expressed the need of it.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Andrea Balla) for the series “Minimally Invasive Approach for the Treatment of Gastro-esophageal Reflux Disease” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-65). The series “Minimally Invasive Approach for the Treatment of Gastro-esophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflusx disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20. [Crossref] [PubMed]
- Becher A, El-Serag H. Systematic Review: the association between symptomatic response to proton pump inhibitors and health related quality of life in patients with gastro-esophageal reflux disease. Aliment Pharmacol Ther 2011;34:618-27. [Crossref] [PubMed]
- Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2005;54:710-7. [Crossref] [PubMed]
- Shaker R, Castell DO, Schoenffeld PS, et al. Nightime heartburn is an under-appreciate clinical problem that impacts sleep and daytime function: the results of a gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol 2003;98:1487-93. [Crossref] [PubMed]
- Mizuta A, Adachi K, Furuta K, et al. Different sex related influences of eating habits on the prevalence of reflux esophagitis in Japan. J Gastroenterol Hepatol 2011;26:1060-4. [Crossref] [PubMed]
- Pace F, Negrini C, Wiklund I, et al. Quality of life in acute and maintenance treatment of non-erosive and mild erosive gastrooesophageal reflux disease. Aliment Pharmacol Ther 2005;22:349-56. [Crossref] [PubMed]
- Velanovich V. Quality of life and severity of symptoms in Gastro-oesophageal reflux disease: a clinical review. Eur J Surg 2000;166:516-25. [Crossref] [PubMed]
- Robinson M, Fitzgerald S, Hegedus R, et al. Onset of symptom relief with rabeprazole: a community-based, open-label assessment of patients with erosive oesophagitis. Aliment Pharmacol Ther 2002;16:445-54. [Crossref] [PubMed]
- Richter JE. Long-term management of gastroesophageal reflux disease and its complications. Am J Gastroenterol 1997;92:30S-34S; discussion 34S-35S. [PubMed]
- Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol 2016;51:751-67. [Crossref] [PubMed]
- Wileman SM, McCann S, Grant AM, et al. Medical versus surgical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev 2010.CD003243. [Crossref] [PubMed]
- Johannessen R, Petersen H, Olberg P, et al. Airway symptom and sleeping difficulties in operated and non-operated patients with gastroesophageal reflux disease. Scand. J. Gastroenterol 2012;47:762-9. [Crossref] [PubMed]
- Zaninotto G, Parante P, Salvador R, et al. Long-term follow-up of Barrett’s epithelium: medical versus antireflux surgical therapy. J Gastrointest Surg 2012;16:7-14; discussion 14-5. [Crossref] [PubMed]
- Brillantino A, Schettino M, Torelli F, et al. Laparoscopic Nissen-Rossetti fundoplication is a safe and effective treatment for both acid and bile gastroesophageal reflux in patients poorly responsive to proton pump inhibitor. Surg Innov 2011;18:387-93. [Crossref] [PubMed]
- Dallemagne B, Weerts J, Markiewicz S, et al. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc 2006;20:159-65. [Crossref] [PubMed]
- Yates RB, Oelschlager BK. Surgical treatment of gastroesophageal reflux disease. Surg Clin North Am 2015;95:527-53. [Crossref] [PubMed]
- Bonavina L, Saino GI, Bona D, et al. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg 2008;12:2133-40. [Crossref] [PubMed]
- Bonavina L, DeMeester T, Fockens P, et al. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg 2010;252:857-62. [Crossref] [PubMed]
- Instructions for use manual of LINX Reflux Management System (Torax Medical).
- Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013;368:719-27. [Crossref] [PubMed]
- Schizas D, Mastoraki A, Papoutsi E, et al. LINX® reflux management system to bridge the "treatment gap" in gastroesophageal reflux disease: A systematic review of 35 studies. World J Clin Cases 2020;8:294-305. [Crossref] [PubMed]
- Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus 2007;20:130-4. [Crossref] [PubMed]
- Schlottmann F, Herbella FA, Allaix ME, et al. Surgical Treatment of Gastroesophageal Reflux Disease. World J Surg 2017;41:1685-90. [Crossref] [PubMed]
- Zhang H, Dong D, Liu Z, et al. Revaluation of the efficacy of magnetic sphincter augmentation for treating gastroesophageal reflux disease. Surg Endosc 2016;30:3684-90. [Crossref] [PubMed]
- Aiolfi A, Asti E, Bernardi D, et al. Early results of magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: Systematic review and meta-analysis. Int J Surg 2018;52:82-8. [Crossref] [PubMed]
- Guidozzi N, Wiggins T, Ahmed AR, et al. Laparoscopic magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: systematic review and pooled analysis. Dis Esophagus 2019;32:doz031. [Crossref] [PubMed]
- Chen MY, Huang DY, Wu A, et al. Efficacy of Magnetic Sphincter Augmentation versus Nissen Fundoplication for Gastroesophageal Reflux Disease in Short Term: A Meta-Analysis. Can J Gastroenterol Hepatol 2017;2017:9596342. [Crossref] [PubMed]
- Lipham JC, Taiganides PA, Louie BE, et al. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis Esophagus 2015;28:305-11. [Crossref] [PubMed]
- Smith CD, Ganz RA, Lipham JC, et al. Lower Esophageal Sphincter Augmentation for Gastroesophageal Reflux Disease: The Safety of a Modern Implant. J Laparoendosc Adv Surg Tech A 2017;27:586-91. [Crossref] [PubMed]
- Asti E, Siboni S, Lazzari V, et al. Removal of the Magnetic Sphincter Augmentation Device: Surgical Technique and Results of a Single-center Cohort Study. Ann Surg 2017;265:941-5. [Crossref] [PubMed]
- Warren HF, Brown LM, Mihura M, et al. Factors influencing the outcome of magnetic sphincter augmentation for chronic gastroesophageal reflux disease. Surg Endosc 2018;32:405-12. [Crossref] [PubMed]
- Hawasli A, Sadoun M, Meguid A, et al. Laparoscopic placement of the LINX® system in management of severe reflux after sleeve gastrectomy. Am J Surg 2019;217:496-9. [Crossref] [PubMed]
- Broderick RC, Smith CD, Cheverie JN, et al. Magnetic sphincter augmentation: a viable rescue therapy for symptomatic reflux following bariatric surgery. Surg Endosc 2020;34:3211-5. [Crossref] [PubMed]
- Telem DA, Wright AS, Shah PC, et al. SAGES technology and value assessment committee (TAVAC) safety and effectiveness analysis: LINX® reflux management system. Surg Endosc 2017;31:3811-26. [Crossref] [PubMed]
- Rona KA, Reynolds J, Schwameis K, et al. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc 2017;31:2096-102. [Crossref] [PubMed]
- Buckley FP 3rd, Bell RCW, Freeman K, et al. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc 2018;32:1762-8. [Crossref] [PubMed]
- Ayazi S, Chowdhury N, Zaidi AH, et al. Magnetic sphincter augmentation (MSA) in patients with hiatal hernia: clinical outcome and patterns of recurrence. Surg Endosc 2020;34:1835-46. [Crossref] [PubMed]
- Reynolds JL, Zehetner J, Nieh A, et al. Charges, outcomes, and complications: a comparison of magnetic sphincter augmentation versus laparoscopic Nissen fundoplication for the treatment of GERD. Surg Endosc 2016;30:3225-30. [Crossref] [PubMed]
- Ayazi S, Zaidi AH, Zheng P, et al. Comparison of surgical payer costs and implication on the healthcare expenses between laparoscopic magnetic sphincter augmentation (MSA) and laparoscopic Nissen fundoplication (LNF) in a large healthcare system. Surg Endosc 2020;34:2279-86. [Crossref] [PubMed]
Cite this article as: Botteri E, Molfino S, Caprioli M, Turolo C, Vettoretto N. New technology minimally invasive for the treatment of gastro-esophageal reflux disease: LINX. Laparosc Surg 2021;5:8.


