
Laparoscopic liver surgery for colorectal liver metastases—a narrative review of the recent literature
Introduction
Colorectal cancer (CRC) represents the third most frequent type of cancer worldwide, with a global incidence of about 1.8 million cases per year. According to approximately 700,000 cancer-related deaths per year, CRC is the fourth leading cause worldwide (1). The most important cause of death are metastases of the liver, which is the most common site for hematogenous metastases in CRC.
Over the last decades minimally invasive surgery has become an integral part of abdominal surgery. In contrast to surgical resection for colon cancer where laparoscopic resection is well established (2), there are still concerns and hesitations towards laparoscopic liver resection despite the first case was performed already 30 years ago (3). However, a recent study showed a substantial increase of laparoscopic liver resections for malignant lesion (4).
This review aims at providing a summary of the contemporary and most recent literature on laparoscopic resection of colorectal liver metastases (LM). A short overview regarding current treatment strategies for colorectal LM will be followed by sections presenting short- and long-term outcomes following laparoscopic resection of colorectal LM and discussing potential limitations of the available data. Emphasis will be put on recent published retrospective data using propensity score adjustments and on prospective randomized trials.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/ls-20-106).
CRC—LM incidence
The incidence of LM comes up to 50%. Approximately 15–25% of patients have synchronous LM at the time of primary diagnosis, and another 15–25% will develop metachronous LM later on, especially during the first five years following radical resection of the primary tumor (5). Patients with untreated LM have an abysmal prognosis with approximately seven months median overall survival with best supportive care (6). However, in patients after successful surgical resection of, both, primary tumor and LM a median overall survival of up to 55 months and a 5-year overall survival of up to 57% are possible in combination with modern systemic chemotherapy and immunotherapy (7,8).
CRC—multimodal treatment (focus on LM and curative approach)
The traditional treatment of CRC without LM is simple: radical resection of the primary tumor and in case of lymph node metastases adjuvant chemotherapy. For patients with LM, however, a lot of different treatment options are available depending on the time of occurrence of LM (synchronous vs. metachronous) and the resectability of the LM (resectable vs. potentially resectable vs. not resectable) (5,9). The synchronous disease is currently associated with a less favorable prognosis compared to metachronous tumor recurrence (10). Importantly, every patient with CRC LM should be discussed in a multidisciplinary tumor-board to guarantee an optimal individual treatment concept. This includes even less common treatment modalities like ALPPS (associated liver partition and portal vein ligation for staged hepatectomy), liver transplantation, radiofrequency ablation (RFA), microwave ablation, transarterial chemoembolization (TACE), transarterial radioembolization or hepatic intraarterial chemotherapy (5,9). It has been proven that discussion and decision making in the tumor-board leads to improved surgical resection rate, superior overall survival, and better quality of life (11,12).
Surgical resection of LM is still considered as the essential therapy to achieve long-term survival (7). Unfortunately, a majority of patients (up to 75%) are not suitable for liver surgery because of unresectable disease, significant comorbidities, poor liver function, or insufficient remnant liver volume (9). The primary treatment goal in these patients is to prolong overall survival and maintain quality of life with systemic chemotherapy (based on fluoropyrimidines, oxaliplatin and irinotecan) and targeted immunotherapies (especially bevacizumab, cetuximab) depending on biomarker expression (in particular RAS, BRAF mutation status) and primary tumor site (right vs. left hemicolon) (1). Patients with initially unresectable but potentially resectable LM should attempt conversion chemotherapy to minimize the size of the metastases. However, appropriate downstaging and resectability can be achieved only in approximately 15% of patients, amongst others, because chemotherapy-associated liver toxicity significantly limits the effective duration of pre-operative chemotherapy (usually three months) (13,14). The continuous development of new, more efficient systemic treatments has been paralleled by surgical and interventional innovations which allowed to stretch the boundaries of liver resection for colorectal LM. The paper of Adam et al. describing the concept of two-stage hepatectomy (TSH) for patients initially deemed unsuitable for surgical resection due to bilobar metastases set a milestone in the field. In these patients, the liver lobe with the lower disease burden is cleared by atypical resections followed by a major liver resection in the second stage. Adjuvant treatments like chemotherapy can be combined to decrease drop-out rates due to tumor progression (15). In the case of marginal remnant liver volume (approximately <30%), portal vein embolization represents an established standard procedure to induce the growth of the future liver remnant and allows hepatic resection unless tumor progression occurs, the main dropout-reason (16,17). These two procedures can be combined by, e.g., embolizing the contralateral portal vein of the future liver remnant if the second stage resection exceeds 60% of the liver volume.
The ALPPS procedure represents another surgical tool. In contrast to the TSH the major surgical procedure is represented by the first stage where intraoperative ligation of the portal vein is combined by parenchymal transection without compromising arterial inflow, venous outflow, and the biliary ducts. The second stage is represented by transection of the artery, the vein and the biliary ducts and the recovery of the diseased liver specimen (18). Due to reported high morbidity and mortality rates many technical modifications have been adopted, including hybrid approaches including interventional and laparoscopic techniques (19). Last but not least, the field of transplant oncology is expanding. While until recently colorectal LM represented an absolute contraindication for liver transplantation, reports from the Oslo group show overall 5-year survival rates of up to 100% after liver transplantation in a selected group of patients (20).
The treatment of patients with synchronous LM consists of resection of both the primary tumor and the LM combined with systemic therapy, either in terms of a simultaneous approach, a traditional staged approach (“colorectal-first”), or a reverse staged approach (“liver-first”) (10). The oncologic outcome of these three treatment strategies is similar. The optimal sequence of surgery and systemic therapy is still under debate, and it depends on different variables like patient symptoms (e.g., bleeding and obstruction of the primary tumor) and tumor burden (number, diameter, and localization of LM) (21). The combined approach with simultaneous resection appears attractive from the patient's perspective, but exhibits a prolonged operative time, higher postoperative morbidity (up to 36%) and higher mortality (up to 8%) (22). Thus, many surgeons prefer the staged procedures, especially the traditional colorectal-first concept, which should decrease the risk of new metastases and prevent the development of colorectal complications (10). On the other hand, the most important argument for the liver-first approach is the immediate start with a systemic treatment by means of upfront chemotherapy before hepatic resection, since response to neoadjuvant chemotherapy has been shown to be one of the strongest predictors of long-term survival and recurrence (1,23). Following successful resection of LM, the optimal postoperative treatment strategy (e.g., adjuvant chemotherapy) is not well-defined, in any case close surveillance is recommended.
The role of laparoscopic liver surgery
Minimally invasive surgery offers a range of benefits for patients, including less surgical trauma, less postoperative pain, earlier time to recovery ending in reduced length of hospital stay. However, while minimally invasive techniques as for cholecystectomy or hernia repair found its way into clinical practice quickly, the distribution of laparoscopic liver resection struggled to find its place (24). Hepatectomy per se is a demanding procedure with the risk of bleeding or bile leakage; hence both open and laparoscopic skills are necessary to be able to perform these procedures safely. The complexity of liver resections varies considerably, depending on the segments that need to get resected. Kawaguchi et al. defined three groups of difficulty (25). Herein, he included in group 1 wedge resections and left lateral segmentectomy, in group 2 anterolateral segmentectomy and left hepatectomy and in group 3 posterosuperior and right posterior resections, right hepatectomy and central resections. Ban et al. (26). based their difficulty grades on pre-operative factors and established a 10-level index with recommendations for surgeons starting with that type of surgery. He also recommended a minimum caseload of each kind of resection. Highly complex resections (Index 10) should only be performed by experienced surgeons who have done more than 50 laparoscopic hepatectomies. The significant advantage of this classification—that might be used as a roadmap for training—is the inclusion of tumor and patient-related factors that can easily be applied before surgery.
In the last 12 years, three international consensus statements in laparoscopic liver resection have been published, reflecting the ongoing and exponential growth of this technique. The first consensus meeting was held in 2008 and is formerly known as the Louisville statement (27). The focus at that time was on the feasibility of laparoscopic liver resection. Evidence has since then gradually developed, leading to the second consensus conference in Morioka in 2014 (28). In this second meeting, recommendations for safe implementation were developed based on data comparing open with laparoscopic hepatectomies. Finally, future directions and technical issues were discussed. In 2017, only three years later, the Southampton Consensus was published by Abu Hilal et al. (29) this conference aimed to provide clinical practice guidelines for safe implementation for experienced as well as less experienced surgeons and centers. In total, 674 publications were reviewed to answer five sections and several topics that affect clinical practice—indications, patients and complex diseases, procedures, technique, and implementation. Notably, the consensus of 95% in each discussed topic was reached among laparoscopic and senior conventional liver surgeons. The main outcomes that were seen throughout most of the comparative studies are less blood loss, shorter hospital stay, and lower morbidity. Thus, the consensus was that the standard approach for low grade complexity resections (e.g., left lateral sectionectomy) should be laparoscopic. With increasing experience, even complex procedures as re-do operations, larger lesions, and extended resections can be performed laparoscopically, even in high-risk patients.
Short-term results
Retrospective studies
Several studies have focused on the feasibility and safety of laparoscopic liver resections compared to open approaches, especially in the case of colorectal LM (30-32). With the increasing utilization of minimally invasive surgery, a growing body of mostly retrospective data has emerged during the last decade. They present laparoscopic liver resection as safe and as having significant benefits in terms of postoperative morbidity and functional recovery compared to open procedures (33-38). A meta-analysis by Xie et al. demonstrated that laparoscopic liver resection is a valid alternative to open liver resection in the treatment of selected cases. Postoperative mortality was equal in both groups, but overall morbidity was up to 50% less in the laparoscopic study group (39). Several other groups published, that blood transfusion and intraoperative blood loss was reduced in minimal invasive liver surgery. For example, Schiffmann et al. described a significantly less transfusion rate in the laparoscopic group (9.9% vs. 19.8%, P=0.004) as well as less intraoperative blood loss (262 vs. 385 mL, P=0.049) (40). The reason for this reduction seems to be attributed to two main factors. On the one hand, there is the hemostatic effect obtained by the pneumoperitoneum, on the other hand, the magnification achieved with laparoscopy enables excellent control of small intrahepatic vascular structures and thus contributes to limit bleeding related to parenchymal dissection. Intraoperative ultrasonography, ultrasonic dissection, microwave coagulators, laparoscopic CUSA, and vascular staplers have also additionally simplified laparoscopic liver resections (41-48). Another main advantage of laparoscopic liver resection is the observed improved postoperative patient comfort, including less need for intensive care unit stay, less postoperative narcotic requirement, and shorter postoperative length of hospital stay (40,43-46). This aspect is confirmed by the observation of Tohme et al., who reported that the time from surgery to postoperative chemotherapy was much shorter in laparoscopic approaches compared to open (49). Furthermore, modern concepts of enhanced recovery after surgery programs combined with minimal invasive resection magnify these effects, which might even positively affect long-term outcomes (50). The reported longer operation time compared to open procedures does not seem to have an impact on clinical outcomes (42).
Prospective studies
In the field of laparoscopic liver resection for colorectal LM, two main prospective randomized controlled trials reporting short-term results have been recently published.
The OSLO-COMET trial (OSLO Randomized Laparoscopic Versus Open Liver Resection for Colorectal Liver Metastases Study) was the first prospective randomized controlled trial reporting that for patients requiring parenchymal-preserving liver resection for colorectal LM, the laparoscopic approach was associated with fewer postoperative complications. Of 280 enrolled patients, more than half of the cases were operated minimally invasive. The rate of severe complications was 19% in the laparoscopic arm versus 31% in open settings. There were no differences in operation time, intraoperative blood loss, and blood transfusions. A shorter stay on the recovery ward, as well as less postoperative morphine equivalents, were observed in the laparoscopic group. Reoperations and readmission within 30 days as well as 90-day mortality were comparable with open procedures (51).
The second published randomized controlled trial is the LapOpHuva study. This study included almost 100 patients in each study group. The data confirmed the results of the OSLO-COMET trial. Even though describing a generally lower incidence of severe complications, the authors confirmed the benefits of laparoscopy in this regard (23.7% complication in the open resection group versus 11.5% in the laparoscopic resection group). Again, operation time, blood loss and postoperative mortality were comparable between both groups (52).
Patients included in the OSLO-COMET trial were also assessed regarding their postoperative quality of life. Of note, patients assigned to laparoscopic surgery reported better postoperative health-related quality of life compared to those assigned to open surgery. For role limitations caused by physical health impairments, patients in the laparoscopic group reported better scores up to 4 months after surgery (53). These results are coherent with an earlier study published by the same group on a small group of patients of the OSLO-COMET trial. The authors observed that laparoscopic liver resection is also associated with reduced inflammatory response compared to open resection (54). These aspects might be of relevance in the context of multimodal treatment strategies where the patients have to get ready as fast as possible to start, e.g., adjuvant systemic treatment. Besides, patients with colorectal LM often need repeat procedures because of recurrence. Reducing trauma and discomfort following laparoscopic liver resection may improve the tolerance and acceptance for reoperations.
A special mention deserves the ORANGE II study. It is an international double-blind randomized controlled trial comparing laparoscopic versus open left lateral sectionectomy with regard to functional recovery. The study was stopped prematurely due to the slow accrual of patients. Rather than pointing at the missing differences between the open and the laparoscopic group, it is essential to highlight that the main reason for recruitment failure was surgeons' preference. It seems that already 10 years ago, when the study started recruiting patients, laparoscopic left lateral sectionectomy was considered to be at least equal to the open procedure (55).
Table 1 reports relevant single- and multicenter studies regarding short-term outcomes published in the last 5 years [Table 1 (33-36,51-53,56)].
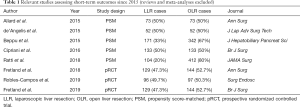
Full table
Long-term results
As for all fields in surgical oncology, good short-term outcomes should not sacrifice long-term, oncologic outcomes. Hepatic resection is currently the only evidence-based potential curative treatment for patients with colorectal LM resulting in 5-year survival of more than 50%. It has already been extensively shown that long-term outcomes after minimally invasive colon cancer resection are equivalent to those following open resection (2). In contrast to publications focusing on short-term results, the amount of work describing mid- and long-term outcomes following laparoscopic liver resection is still underrepresented. However, there is now an increasing amount of literature supporting laparoscopic resection of LM even from an oncological point of view.
Retrospective studies
The first studies analyzing long-term oncologic results between laparoscopic and open liver resections were published already ten years ago. However, they are hampered by selection bias, which makes interpretation difficult. Significantly higher numbers of cleared resection margins and better cancer-specific survival of patients having had a laparoscopic resection were rather the result of comparing groups being treated in different eras—with different systemic treatment modalities and distinct tumor burden—than the consequence of a real advantage of the laparoscopic technique (57). To address this shortcoming and in the light of missing prospective randomized trials, studies increasingly relied on propensity score adjustments. Balancing patient groups across known risk factors and confounders, which may result in selection bias, has been increasingly used in surgical literature in the last decade. Recent studies define this method to represent a robust alternative to randomized controlled trials provided that covariates on which matching is based are clearly presented in the manuscript (58,59).
Resection margin
Negative resection margins are seen as a surrogate marker for radical, oncological resection and hence also for long-term survival. Still, for colorectal LM, the impact of tumor-free resection margins on overall survival is a matter of debate. While some data suggest that overall survival is directly related to the width of tumor-free margins (60), others argument that tumor biology is more important (61).
In general, for both approaches, the incidence of positive resection margins ranges between 5% and 28%. However, rather than addressing differences between laparoscopic and open approaches, it rather reflects residual bias like demanding tumor location. Besides, there is heterogeneity in defining negative resection margins (0 mm versus <1 mm), which makes a comparison between different studies more difficult (40,46,56). In a recent publication comparing open and laparoscopic surgery with more than 600 patients per group, there was no difference between laparoscopy and open resection regarding the incidence of positive resection margins (<1 mm). In both groups, positive resection margins were associated with decreased recurrence-free survival (RFS). Interestingly, only in the open surgery group, it was also associated with a decreased overall survival (OS). A possible explanation might rely on the observed higher number of re-hepatectomies in the laparoscopic group (62). Similarly, Montalti et al. report that positive resection margins do not affect OS following laparoscopic liver resection. In contrast, recurrence-free survival is affected. Again, the possibility of a repeat hepatectomy might explain this apparent discrepancy (63). The importance of salvageability by repeat hepatectomy is also shown in a recent analysis comparing laparoscopic major and parenchyma sparing hepatectomies. Despite similar negative resection margin incidence, there was a tendency towards better OS in the parenchyma sparing group which was associated also with a higher incidence of re-hepatectomies (64).
Recurrence free survival and overall survival
Even though there is agreement about the importance of negative resection margins—with some authors emphasizing the importance of achieving at least 1 mm negative margins—the crucial outcome reflecting the oncological equivalence between the two techniques remain RFS and OS. Since patients can be cured following resection of their hepatic metastases and since resection margins do not exclusively reflect OS, some leaders in the field propose to focus on 5- and 10-year milestones to better define oncologic outcomes (65,66).
Looking at the retrospective studies of the last decade, laparoscopic liver resections show comparable results regarding 5-year RFS. For both approaches, 5-year RFS ranges between 20% and almost 40%. The same pattern can also be observed for 5-year OS. Here, the data range between 40% and more than 70%, again equally distributed in both groups. While this confirms feasibility as well as oncological safety of laparoscopic liver resection for colorectal LM, it also points to the heterogeneity of the available data. Paradigmatically, in a recent multicenter study, the range of laparoscopic resections performed in the included centers ranged from 1.4% to 100% (35) .However, throughout the current literature, the reported data support the implementation of the laparoscopic approach for colorectal LM. Results are also confirmed using different matching strategies. Stratifying patients with colorectal LM according to the pre-operative score of the Japanese Society of Hepato-Biliary-Pancreatic Society showed no differences in RFS and OS between open and laparoscopic surgery in a multicenter Japanese study (67). Similarly, the importance of matching patients in comparative studies according to, e.g., surgical “era”, tumor burden, and perioperative systemic treatment, confirm these findings. Of note, the comparison of unbalanced data results in significantly better RFS and OS in the laparoscopic groups (34,36). This reality distortion of the unbalanced data reflects the steady development of new systemic treatments, the later implementation of laparoscopic surgery compared to open liver surgery, and the meticulous case selection in the early laparoscopic era. These disbalance between the two groups should gradually disappear in future studies as the substantial improvements in short-term results lead to a broader indication for laparoscopic resection, especially in high volume centers (68).
Prospective studies
The already mentioned OSLO-COMET (51) and the LapOpHuva (52) studies are, so far, the only two randomized controlled trials comparing laparoscopic and open resection of colorectal LM and addressing oncological issues. Concerning long-term data, it is essential to highlight some differences. The OSLO-COMET trial had an enrollment phase of four years (2012 to 2016), resulting in homogeneous study groups and focusing on the parenchyma sparing concept, including only patients requiring resection of less than three consecutive segments. The LapOpHuva trial enrolled patients over a time frame of more than 10 years, and it also included major hepatectomies (7% in the open and 11% in the laparoscopic group). Of note, 400 (OSLO-COMET trial) and 50 (LapOpHuva trial) laparoscopic liver resections were performed before starting with patient enrollment. In both studies, there was no difference regarding resection margins between open and laparoscopic approach. Similarly, 5-year RFS and OS in LapOpHuva trial was 23.9% and 47.4% for the open group and 22.7% and 49.3% for the laparoscopic group, showing no significant differences. So far, the OSLO-COMET trial released only mid-term outcomes after completion of 36 months follow-up. Median RFS and OS in the laparoscopic and in the open group was 19 months (range, 10–27) and 16 months (range, 11–21), and 80 months (range, 52–108) and 81 months (range, 46–120), respectively. No differences were seen (69). Interestingly, a >1 mm negative margin was achieved in the OSLO-COMET in 71%, and in the LapOpHuva in approximately 90%. Both studies achieved negative margins with <1 mm disease-free tissue in more than 90%. Similar results regarding RFS and OS questions the necessity of wider margins in parenchyma sparing liver resections.
Table 2 reports relevant single- and multicenter studies regarding oncologically relevant outcomes published in the last 5 years (33-36,52,56,62,70,71).

Full table
TSH and ALPPS
Data on more complex laparoscopic procedures like TSH and ALPPS are less present in the literature and reflect the activity of highly specialized centers. In a recent retrospective propensity score matched study involving two French tertiary centers, short term results showed similar advantages to those for “standard” laparoscopic liver resections. Also oncologic outcomes were not inferior to the open procedure. The higher incidence of repeat hepatectomies in the laparoscopic group might suggest even a better long-term outcome in the laparoscopic group. However, 5 year overall and recurrence free survival data are still missing (72).
Similarly, randomized trials analyzing the impact of minimal invasive surgery in ALPPS are missing. A recent systematic review identified only 15 papers dealing with this topic reporting in total 27 patients. Another one published 2020 reports 46 cases. Even though mortality and morbidity rates were lower compared to the open procedures results should be considered with caution since there were important differences between open and laparoscopically operated patients with regard to underlying pathologies as well as to the extent of the resections (73,74).
Alternative minimally invasive approaches
Without going to much beyond the topic of this review it is just fair to mention two other minimally invasive local treatment options for colorectal LM which will surely become more present in the near future.
One is robotic surgery which achieved significant advances not only with regard to the feasibility of liver resections but also with regard to the possibility of implementing artificial intelligence in robotic platforms (75,76). Even though this opens fascinating scenarios with substantially decreased morbidity and mortality rates, and better ergonomics for surgeons, this type of surgery might be burdened by its high costs rather than by its learning curve. The emergence of different robotic platforms could represent an important step for a wider accessibility (77).
Encouraged by good short- as well as long-term results following atypical laparoscopic resections percutaneous approaches like stereotactic RFA and microwave ablation have recently emerged as valid, curative alternatives to liver resections. Especially in frail patients or in situations of centrally located, single metastases percutaneous approaches offer several short- and mid-term advantages (78-80). So far, prospective randomized controlled trial data on long-term outcomes are missing.
Conclusions
This review confirms the data of two recent meta-analyses comparing laparoscopic and open liver resection for colorectal LM (81,82). The laparoscopic approach in resectable colorectal LM not only results in better short-term outcomes like increased patient comfort and reduced perioperative morbidity and mortality, but it is also at least equivalent regarding oncological outcomes like negative resection margins, 5-year RFS and OS.
Still, it has to be considered that retrospectively as well as prospectively collected data come from highly experienced centers. General application of laparoscopic liver resection and reproduction of these excellent data crucially relies on careful patient selection and on the definition of the surgeons’ comfort zone in laparoscopic surgery. Nevertheless, step by step implementation of the laparoscopic approach should be supported, beginning with parenchyma sparing resections in “easy” positions. Major resection should still be performed in highly experienced centers.
Last but not least, real 5-year survival data of prospective studies are still missing. These data are eagerly awaited. They might provide the needed high evidence of a suggested long-term advantage of laparoscopic over open surgery in colorectal LM.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Robert Sucher and Elisabeth Sucher) for the series “Minimally Invasive Liver Surgery” published in Laparoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/ls-20-106
Peer Review File: Available at http://dx.doi.org/10.21037/ls-20-106
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-106). The series “Minimally Invasive Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rehman AH, Jones RP, Poston G. Prognostic and predictive markers in liver limited stage IV colorectal cancer. Eur J Surg Oncol 2019;45:2251-6. [Crossref] [PubMed]
- Buunen M, Veldkamp R, Hop WC, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44-52. [Crossref] [PubMed]
- Biertho L, Waage A, Gagner M. Laparoscopic hepatectomy. Ann Chir 2002;127:164-70. [Crossref] [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Ren L, Zhu D, Benson AB, et al. Shanghai international consensus on diagnosis and comprehensive treatment of colorectal liver metastases (version 2019). Eur J Surg Oncol 2020;46:955-66. [Crossref] [PubMed]
- Norén A, Eriksson HG, Olsson LI. Selection for surgery and survival of synchronous colorectal liver metastases; a nationwide study. Eur J Cancer 2016;53:105-14. [Crossref] [PubMed]
- Giuliante F, Ardito F, Vellone M, et al. Role of the surgeon as a variable in long-term survival after liver resection for colorectal metastases. J Surg Oncol 2009;100:538-45. [Crossref] [PubMed]
- Jones RP, Kokudo N, Folprecht G, et al. Colorectal Liver Metastases: A Critical Review of State of the Art. Liver Cancer 2016;6:66-71. [Crossref] [PubMed]
- Dörr NM, Bartels M, Morgul MH. Current Treatment of Colorectal Liver Metastasis as a Chronic Disease. Anticancer Res 2020;40:1-7. [Crossref] [PubMed]
- Lillemoe HA, Vauthey JN. Surgical approach to synchronous colorectal liver metastases: staged, combined, or reverse strategy. Hepatobiliary Surg Nutr 2020;9:25-34. [Crossref] [PubMed]
- Lan YT, Jiang JK, Chang SC, et al. Improved outcomes of colorectal cancer patients with liver metastases in the era of the multidisciplinary teams. Int J Colorectal Dis 2016;31:403-11. [Crossref] [PubMed]
- Wanis KN, Pineda-Solis K, Tun-Abraham ME, et al. Management of colorectal cancer with synchronous liver metastases: impact of multidisciplinary case conference review. Hepatobiliary Surg Nutr 2017;6:162-9. [Crossref] [PubMed]
- Wicherts DA, de Haas RJ, Adam R. Bringing unresectable liver disease to resection with curative intent. Eur J Surg Oncol 2007;33:S42-51. [Crossref] [PubMed]
- Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008;247:118-24. [Crossref] [PubMed]
- Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777-85. [Crossref] [PubMed]
- Shimada S, Kamiyama T, Yokoo H, et al. Hepatic hypertrophy and hemodynamics of portal venous flow after percutaneous transhepatic portal embolization. BMC Surg 2019;19:23. [Crossref] [PubMed]
- Ironside N, Bell R, Bartlett A, et al. Systematic review of perioperative and survival outcomes of liver resections with and without preoperative portal vein embolization for colorectal metastases. HPB (Oxford) 2017;19:559-66. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Lang H, de Santibañes E, Schlitt HJ, et al. 10th Anniversary of ALPPS-Lessons Learned and quo Vadis. Ann Surg 2019;269:114-9. [Crossref] [PubMed]
- Dueland S, Grut H, Syversveen T, et al. Selection criteria related to long-term survival following liver transplantation for colorectal liver metastasis. Am J Transplant 2020;20:530-7. [Crossref] [PubMed]
- Kelly ME, Spolverato G, Lê GN, et al. Synchronous colorectal liver metastasis: a network meta-analysis review comparing classical, combined, and liver-first surgical strategies. J Surg Oncol 2015;111:341-51. [Crossref] [PubMed]
- Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol 2007;14:3481-91. [Crossref] [PubMed]
- Mentha G, Majno PE, Andres A, et al. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg 2006;93:872-8. [Crossref] [PubMed]
- Guilbaud T, Birnbaum DJ, Berdah S, et al. Learning Curve in Laparoscopic Liver Resection, Educational Value of Simulation and Training Programmes: A Systematic Review. World J Surg 2019;43:2710-9. [Crossref] [PubMed]
- Kawaguchi Y, Fuks D, Kokudo N, et al. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg 2018;267:13-7. [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Gumbs AA, Bar-Zakai B, Gayet B. Totally laparoscopic extended left hepatectomy. J Gastrointest Surg 2008;12:1152. [Crossref] [PubMed]
- Nomi T, Fuks D, Govindasamy M, et al. Risk factors for complications after laparoscopic major hepatectomy. Br J Surg 2015;102:254-60. [Crossref] [PubMed]
- Ito K, Ito H, Are C, et al. Laparoscopic versus open liver resection: a matched-pair case control study. J Gastrointest Surg 2009;13:2276-83. [Crossref] [PubMed]
- de'Angelis N, Eshkenazy R, Brunetti F, et al. Laparoscopic versus open resection for colorectal liver metastases: a single-center study with propensity score analysis. J Laparoendosc Adv Surg Tech A 2015;25:12-20. [Crossref] [PubMed]
- Beppu T, Wakabayashi G, Hasegawa K, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:711-20. [Crossref] [PubMed]
- Allard MA, Cunha AS, Gayet B, et al. Early and Long-term Oncological Outcomes After Laparoscopic Resection for Colorectal Liver Metastases: A Propensity Score-based Analysis. Ann Surg 2015;262:794-802. [Crossref] [PubMed]
- Cipriani F, Rawashdeh M, Stanton L, et al. Propensity score-based analysis of outcomes of laparoscopic versus open liver resection for colorectal metastases. Br J Surg 2016;103:1504-12. [Crossref] [PubMed]
- Iwahashi S, Shimada M, Utsunomiya T, et al. Laparoscopic hepatic resection for metastatic liver tumor of colorectal cancer: comparative analysis of short- and long-term results. Surg Endosc 2014;28:80-4. [Crossref] [PubMed]
- O'Rourke N, Shaw I, Nathanson L, et al. Laparoscopic resection of hepatic colorectal metastases. HPB (Oxford) 2004;6:230-5. [Crossref] [PubMed]
- Xie SM, Xiong JJ, Liu XT, et al. Laparoscopic Versus Open Liver Resection for Colorectal Liver Metastases: A Comprehensive Systematic Review and Meta-analysis. Sci Rep 2017;7:1012. [Crossref] [PubMed]
- Schiffman SC, Kim KH, Tsung A, et al. Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery 2015;157:211-22. [Crossref] [PubMed]
- Medbery RL, Chadid TS, Sweeney JF, et al. Laparoscopic vs. open right hepatectomy: a value-based analysis. J Am Coll Surg 2014;218:929-39. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Laparoscopic major hepatectomies: current trends and indications. A comparison with the open technique. Updates Surg 2015;67:157-67. [Crossref] [PubMed]
- Castaing D, Vibert E, Ricca L, et al. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg 2009;250:849-55. [Crossref] [PubMed]
- Guerron AD, Aliyev S, Agcaoglu O, et al. Laparoscopic versus open resection of colorectal liver metastasis. Surg Endosc 2013;27:1138-43. [Crossref] [PubMed]
- Inoue Y, Hayashi M, Tanaka R, et al. Short-term results of laparoscopic versus open liver resection for liver metastasis from colorectal cancer: a comparative study. Am Surg 2013;79:495-501. [Crossref] [PubMed]
- Cannon RM, Scoggins CR, Callender GG, et al. Laparoscopic versus open resection of hepatic colorectal metastases. Surgery 2012;152:567-73; discussion 573-4. [Crossref] [PubMed]
- Cipriani F, Shelat VG, Rawashdeh M, et al. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J Am Coll Surg 2015;221:265-72. [Crossref] [PubMed]
- Uchiyama K, Ueno M, Ozawa S, et al. Combined use of contrast-enhanced intraoperative ultrasonography and a fluorescence navigation system for identifying hepatic metastases. World J Surg 2010;34:2953-9. [Crossref] [PubMed]
- Tohme S, Goswami J, Han K, et al. Minimally Invasive Resection of Colorectal Cancer Liver Metastases Leads to an Earlier Initiation of Chemotherapy Compared to Open Surgery. J Gastrointest Surg 2015;19:2199-206. [Crossref] [PubMed]
- Ratti F, Cipriani F, Reineke R, et al. Impact of ERAS approach and minimally-invasive techniques on outcome of patients undergoing liver surgery for hepatocellular carcinoma. Dig Liver Dis 2016;48:1243-8. [Crossref] [PubMed]
- Fretland Å, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Robles-Campos R, Lopez-Lopez V, Brusadin R, et al. Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc 2019;33:3926-36. [Crossref] [PubMed]
- Fretland Å, Dagenborg VJ, Waaler Bjørnelv GM, et al. Quality of life from a randomized trial of laparoscopic or open liver resection for colorectal liver metastases. Br J Surg 2019;106:1372-80. [Crossref] [PubMed]
- Fretland AA, Sokolov A, Postriganova N, et al. Inflammatory Response After Laparoscopic Versus Open Resection of Colorectal Liver Metastases: Data From the Oslo-CoMet Trial. Medicine (Baltimore) 2015;94:e1786 [Crossref] [PubMed]
- Wong-Lun-Hing EM, van Dam RM, van Breukelen GJ, et al. Randomized clinical trial of open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery after surgery programme (ORANGE II study). Br J Surg 2017;104:525-35. [Crossref] [PubMed]
- Ratti F, Fiorentini G, Cipriani F, et al. Laparoscopic vs. Open Surgery for Colorectal Liver Metastases. JAMA Surg 2018;153:1028-35. [Crossref] [PubMed]
- Welsh FK, Tekkis PP, John TG, et al. Open liver resection for colorectal metastases: better short- and long-term outcomes in patients potentially suitable for laparoscopic liver resection. HPB (Oxford) 2010;12:188-94. [Crossref] [PubMed]
- Lonjon G, Boutron I, Trinquart L, et al. Comparison of treatment effect estimates from prospective nonrandomized studies with propensity score analysis and randomized controlled trials of surgical procedures. Ann Surg 2014;259:18-25. [Crossref] [PubMed]
- Lonjon G, Porcher R, Ergina P, et al. Potential Pitfalls of Reporting and Bias in Observational Studies With Propensity Score Analysis Assessing a Surgical Procedure: A Methodological Systematic Review. Ann Surg 2017;265:901-9. [Crossref] [PubMed]
- Sadot E, Groot Koerkamp B, Leal JN, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg 2015;262:476-85; discussion 483-5. [Crossref] [PubMed]
- Hamady ZZ, Lodge JP, Welsh FK, et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg 2014;259:543-8. [Crossref] [PubMed]
- Martínez-Cecilia D, Wicherts DA, Cipriani F, et al. Impact of resection margins for colorectal liver metastases in laparoscopic and open liver resection: a propensity score analysis. Surg Endosc 2021;35:809-18. [Crossref] [PubMed]
- Montalti R, Tomassini F, Laurent S, et al. Impact of surgical margins on overall and recurrence-free survival in parenchymal-sparing laparoscopic liver resections of colorectal metastases. Surg Endosc 2015;29:2736-47. [Crossref] [PubMed]
- Okumura S, Tabchouri N, Leung U, et al. Laparoscopic Parenchymal-Sparing Hepatectomy for Multiple Colorectal Liver Metastases Improves Outcomes and Salvageability: A Propensity Score-Matched Analysis. Ann Surg Oncol 2019;26:4576-86. [Crossref] [PubMed]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575-80. [Crossref] [PubMed]
- Pulitanò C, Castillo F, Aldrighetti L, et al. What defines 'cure' after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB (Oxford) 2010;12:244-9. [Crossref] [PubMed]
- Hasegawa Y, Nitta H, Sasaki A, et al. Long-term outcomes of laparoscopic versus open liver resection for liver metastases from colorectal cancer: A comparative analysis of 168 consecutive cases at a single center. Surgery 2015;157:1065-72. [Crossref] [PubMed]
- Scuderi V, Barkhatov L, Montalti R, et al. Outcome after laparoscopic and open resections of posterosuperior segments of the liver. Br J Surg 2017;104:751-9. [Crossref] [PubMed]
- Fretland ÅA, Aghayan D, Edwin B, et al. Long-term survival after laparoscopic versus open resection for colorectal liver metastases. J Clin Oncol 2019;37. [Crossref]
- Lewin JW, O'Rourke NA, Chiow AKH, et al. Long-term survival in laparoscopic vs. open resection for colorectal liver metastases: inverse probability of treatment weighting using propensity scores. HPB (Oxford) 2016;18:183-91. [Crossref] [PubMed]
- Martínez-Cecilia D, Cipriani F, Shelat V, et al. Laparoscopic Versus Open Liver Resection for Colorectal Metastases in Elderly and Octogenarian Patients: A Multicenter Propensity Score Based Analysis of Short- and Long-term Outcomes. Ann Surg 2017;265:1192-200. [Crossref] [PubMed]
- Okumura S, Goumard C, Gayet B, et al. Laparoscopic versus open two-stage hepatectomy for bilobar colorectal liver metastases: A bi-institutional, propensity score-matched study. Surgery 2019;166:959-66. [Crossref] [PubMed]
- Melandro F, Giovanardi F, Hassan R, et al. Minimally Invasive Approach in the Setting of ALPPS Procedure: a Systematic Review of the Literature. J Gastrointest Surg 2019;23:1917-24. [Crossref] [PubMed]
- Michal K, Sau M, Tamara GMH, et al. A better route to ALPPS: minimally invasive vs. open ALPPS. Surg Endosc 2020;34:2379-89. [Crossref] [PubMed]
- Guerra F, Guadagni S, Pesi B, et al. Outcomes of robotic liver resections for colorectal liver metastases. A multi-institutional analysis of minimally invasive ultrasound-guided robotic surgery. Surg Oncol 2019;28:14-8. [Crossref] [PubMed]
- Oldhafer KJ, Peterhans M, Kantas A, et al. Navigated liver surgery: Current state and importance in the future. Chirurg 2018;89:769-76. [Crossref] [PubMed]
- Teber D, Engels C, Maier-Hein L, et al. Surgery 4.0-are we ready? Urologe A 2020;59:1035-43. [Crossref] [PubMed]
- Shady W, Petre EN, Gonen M, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes--A 10-year Experience at a Single Center. Radiology 2016;278:601-11. [Crossref] [PubMed]
- Shady W, Petre EN, Do KG, et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J Vasc Interv Radiol 2018;29:268-75.e1. [Crossref] [PubMed]
- Schullian P, Putzer D, Silva MA, et al. Stereotactic Radiofrequency Ablation of Liver Tumors in Octogenarians. Front Oncol 2019;9:929. [Crossref] [PubMed]
- Ciria R, Ocaña S, Gomez-Luque I, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc 2020;34:349-60. [Crossref] [PubMed]
- Syn NL, Kabir T, Koh YX, et al. Survival Advantage of Laparoscopic Versus Open Resection For Colorectal Liver Metastases: A Meta-analysis of Individual Patient Data From Randomized Trials and Propensity-score Matched Studies. Ann Surg 2020;272:253-65. [Crossref] [PubMed]
Cite this article as: Braunwarth EM, Perathoner A, Stättner S, Maglione M. Laparoscopic liver surgery for colorectal liver metastases—a narrative review of the recent literature. Laparosc Surg 2021;5:20.


