
Narrative review of 3D navigated stereotactic liver ablation—do we still need a minimally invasive liver surgeon?
Introduction
The ultimate goal of any local treatment in liver cancer is complete tumor eradication to increase patient survival. Also high in the priorities, the treatment should have a low risk of severe complications—as they are associated with a decreased OS—and third, the treatment should have the least possible impact, allowing for early initiation of adjuvant therapies. The latter factors favor the use of minimally invasive techniques, as long as they fulfill the first.
Maximal sparing of functional liver parenchyma and—even more critical—sparing of relevant anatomical structures such as liver veins and hilar structures is key because subsequent re-treatment for recurrent tumor may be necessary. Other important factors include the amount of blood loss during the procedure, procedure-related pain, length of intensive care and hospital stay, recovery time, and late complications.
Within this frame, minimally invasive percutaneous thermal ablation methods seem to be an attractive alternative to surgical resection.
Recent international guidelines already present thermal ablation as valid first-line alternative to surgical resection for singular HCC <2 cm (“very early” HCC, BCLC 0) (1).
However, surgical resection is still recommended as the first-line local curative treatment for any other primary and secondary liver tumors, while percutaneous thermal ablation is reserved for inoperable liver malignancies (2). The reason for that is the unacceptably high local recurrence rate after conventional CT and US-guided thermal ablation.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/ls-20-107).
Limitations of traditional “freehand” CT-, US- and laparoscopic guided thermal ablation
Ultrasound (US) allows for real-time visualization of the probe during puncture. However, this technique is strongly dependent on the practitioner, and many lesions are not sufficiently visible. Besides, probe repositioning during the ablation process is difficult because of limited visibility through the development of gas bubbles.
Many lesions are only visible in contrast-enhanced CT. Therefore, the tumor might not be sufficiently detectable in the native control CT scans that are required during the iterative advancement of the probe into the tumor. This factor might lead to substantial inaccuracies. Besides, many tumor locations require double-angled approaches, which are challenging to perform.
In this narrative review, we will review the published literature on the use of radiofrequency ablation through coaxial (trocar) needles positioned using a stereotactic approach. The aim is to provide the reader with a comprehensive overview of the performance of the technique in different clinical scenarios, with short and long-term data so it can be compared with other published results. Unfortunately, a search in Pubmed and Embase databases, using the terms [liver, ablation, stereotactic and coaxial] gave only publications on this technique from our center. Because of this, in the description of the technique we will explain which advantages we perceive of this technique, in the hope to stimulate adoption by other centers.
Recent data of our group (3) confirmed previous observations (4) that an intraoperatively measured minimal ablative margin (MAM) >5 mm should be achieved to avoid local tumor progression after thermal ablation of HCC. Moreover, in CRLM, a safety margin of at least 1 cm was recommended by an international expert group (5). For the ablation diameter and volume, there are patient-related factors such as perfusion and pathological tissue changes; but it mainly depends on the probe technology. In vivo, the short-diameter of the coagulation zone by using conventional needle-like RFA probes is 1.5 cm. With the latest microwave probes, a short-diameter of 4 cm of ablation can be obtained at most (6). Thus, the maximum sizes of HCCs and CRLM that can be treated with one probe position are 3 and 2 cm, respectively, and that already requires perfect probe positioning.
To treat liver tumors larger than 2–3 cm, multiple overlapping ablation zones in three dimensions must be attained. This task is challenging to achieve by traditional “freehand” ultrasound-/CT-guided and laparoscopic single-probe guidance (7) and accounts for the dissatisfying results in large lesions. In analogy to complex surgical resections—outcomes of these conventionally—guided ablation methods differ sharply between interventionalists with diverging levels of skills (including hand-eye coordination, three-dimensional imagination) and experience.
If multiple needles are planned to create multiple overlapping ablation areas and that planning is executed with consistency, the result is a standardized procedure that is mostly operator-independent. The size-related limitations of ablation can be eliminated, as a theoretical ablation area of any size can be performed reliably. In the following paragraphs, we will explain our technical approach and summarize the clinical evidence from our group and others using similar approaches.
Stereotaxy
Stereotaxy, working on a 3D coordinate system, has been used for decades in neurosurgery for biopsies and tumor treatments. To achieve the maximum accuracy, a stereotactic frame with the medical instruments is fixed to the patient's skull employing screws. The image-based computer-aided calculation of the paths and distances in a Cartesian coordinate system allows precise placement of the instruments inside the patient.
Frame-based stereotaxy, has disadvantages like the limitation of the surgical access routes and the invasive fixation of the frame. These were overcome by the introduction of frameless stereotactic three-dimensional navigation systems in the early nineties, which are now part of the standard equipment in neurosurgical operating rooms. These systems enable users to locate a point within the patient in the 3D coordinate system of a CT or MR image in real-time if the registration between both is accurate. Due to their flexibility, modern navigation systems may be used in various body regions. The software of the navigation systems also allows for the planning of needle trajectories in a cartesian coordinate system. Aiming devices are adjusted according to the virtual pre-/intraoperative plan and facilitate precise percutaneous punctures of virtually every anatomical structure. While the aiming device in passive navigation systems is set manually, robot-assisted systems may carry out these settings (semi-)automatically. For stereotactic and robotic interventions in the liver, control of respiratory motion is an essential prerequisite that can be achieved by disconnection of the endotracheal tube and muscle relaxation during anesthesia (8) or by jet ventilation (9,10) or by the THRIVE anesthesia technique (11,12).
Stereotactic radiofrequency ablation of liver tumors
In 2001 we performed the first in man stereotactic radiofrequency ablation (SRFA) of a malignant liver tumor (13) with intraoperative image fusion for immediate treatment verification by using a commercial neuronavigation system in combination with a novel aiming device, that we originally developed for percutaneous radiofrequency ablation of the Gasserian ganglion in patients with trigeminal neuralgia (14). Since then, we have treated more than 1,000 patients with 4,000 tumors with this technique. Each patient and each tumor are tracked in our prospective SRFA registry, implemented in the hospital information system.
Technique of multi-needle stereotactic radiofrequency ablation (SRFA) with intraoperative image fusion (13,15,16)
In our center, the whole procedure is performed in a dedicated intervention room with a sliding gantry CT (Somatom Sensation, Siemens, Erlangen), which moves on rails between two different rooms that are separated by a large led wall that can be easily opened and closed.
The technique of SRFA (Video 1) was previously described in detail (13,15,16) (Figure 1). In brief, the anesthetized patient is immobilized on the CT table by means of a vacuum mattress. A contrast-enhanced CT in the arterial and portal venous phase is obtained in maximum expiration, which is achieved by disconnection of the endotracheal tube and maximum muscle relaxation. The CT data is transferred to the S8 navigation system (Medtronic Inc., Louisville, KY, USA) by the hospital’s intranet. Access path and ablation planning are performed on the three-dimensional reconstructed CT dataset with the software of the 3D navigation system. If required, preoperative CT/MR/PET/SPECT data may be fused to the intraprocedural CT dataset (17). Fusion is especially helpful if the lesions are not visible in the planning CT.
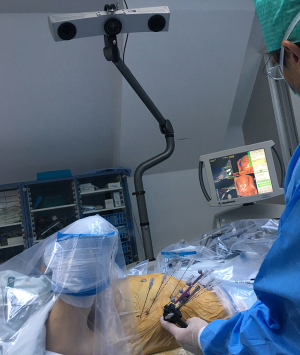
After patient registration with skin fiducials and sterile washing and draping, the Atlas targeting device (Interventional Systems GmbH, Kitzbühel, Austria) is aligned with the virtual trajectories. Coaxial needles are sequentially advanced through the locked aiming device to the preplanned depth in full expiration.
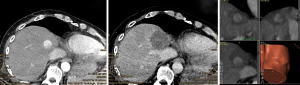
For verification of correct needle placement, a native control CT with the needles in place is superimposed to the planning CT. A 16 g biopsy is obtained, and a maximum of three RFA probes are inserted at a time over the coaxial needles, which serve as placeholders. RF ablation is performed with a unipolar ablation device with a switching controller. After hot probe withdrawal, a contrast-enhanced CT in arterial and portal venous phase is acquired. The superimposition of the control-CT to the planning CT allows checking in three dimensions whether the ablation zones cover the tumors with a sufficient safety margin (Figure 2). In the event of incomplete ablation, the intervention may be continued in the same session by the stereotactic placement of additional coaxial needles with subsequent ablation.
The advantages we perceive from this approach are the following: when the coaxial needles are placed in the planned positions before ablating, they remain in the position relative to the tumor even after the tissue shrinks. Moreover, larger ablation areas can be created by positioning multiple coaxial needles instead of positioning multiple electrodes, which are heavier and more space occupying. This is especially relevant in heavy patients where the space in the CT gantry is limited. Only up to three probes are used per case, which is financially sustainable.
The positioning of coaxial needles also allows for biopsy taking of each tumor. Similar benefits where described by Ishizaka et al. (18) on their communications on the use of coaxial needles for CT guided ablation. As to the thermal technology used, RFA has the benefit—in the case of multiple needle usage—of having a smaller thermosphere, which allows for a more precise tailoring of the ablation area and less risk of sacrificing healthy tissue or delicate structures. This is particularly important when treating lesions in infants (19) or in organs with a small functional reserve.
Respiratory triggering
Respiratory triggering is of utmost importance for stereotactic or robotic coaxial needle placement because the patient is assumed as a rigid body by the navigation system. Thus, the liver has to be at an identical spatial position during each image acquisition and needle placement. The safety of temporary disconnections of the endotracheal tube (ETT) in anesthetized patients was evaluated in 26 patients and revealed an overall mean respiratory motion control error (RMCE) of 1.98±0.93 mm (range, 0.44–4.02 mm) for external targets and 1.41±0.75 mm (range, 0.46–3.18 mm) for internal targets (8).
Targeting accuracy
The targeting accuracy of the stereotactic needle placement was evaluated in 20 patients with 35 liver lesions (20). A total of 145 needles were placed with a mean (± SD) lateral error of 3.6±2.5 mm at the needle tip.
Results after SRFA
Inter-operator performance
To evaluate the reliability of SRFA, the performance between an interventional oncologist with more than >10 years of experience, and a young trainee with only two months of SRFA training was compared (21). Ninety consecutive patients underwent SRFA for 72 primary and 105 secondary liver tumors with a mean size of 2.9 cm (range, 0.5–11 cm). No significant difference was observed between the two operators in terms of technique effectiveness, morbidity, mortality, hospital stay, and local tumor progression.
Histopathological results after SRFA
Recently we reported the effectiveness of SRFA for hepatocellular carcinoma in 97 patients for bridging to liver transplantation (22). A complete histopathological response was accomplished in 183 of 188 nodules (97.3%). Despite the use of SRFA alone, 50 of 52 nodules >3 cm (96.2%) showed complete response in explant histopathology.
Long term results
Intrahepatic cholangiocellular carcinoma (ICC) (23)
In 17 inoperable patients with 52 intrahepatic cholangiocellular carcinomas, ICCs with a mean diameter of 3 cm (0, 5–10 cm) after a median follow-up time of 35 months. The local recurrence rate was 8% and an estimated median OS of 60 months was achieved.
Colorectal liver metastasis (CRLM) (16)
In another study, the long-term results after SRFA of 189 CRLM in 63 consecutive patients from 2005 to 2011 were reported (16). After an average follow-up time of 25 months, the local recurrence rate (LR) was 16%. The median OS was significantly longer in resectable vs. non-resectable patients (27 vs. 58 months, P=0.002) with 1-, 3- and 5-year OS rates of 92%, 66% and 48% in resectable patients. In contrast to the conventional “freehand” RFA, the tumor size had no influence on local recurrence, OS, or DFS.
Breast cancer liver metastases (BCLM) (24)
Recently our group reported the results after SRFA of 64 liver metastases (BCLMs) (including nine lesions >5 cm) in 26 patients, who had progressed under systemic therapy or had discontinued systemic therapy due to side effects. Local recurrence was observed in 5/64 metastases (7.8%), with no significant differences between tumors <3 cm (9.3%), 3–5 cm (0%) and >5 cm (8.3%). The estimated median OS from the first SRFA was 29.3±8.9 months after a median follow-up of 23.1 months.
Melanoma liver metastases (MLM) (25)
From January 2005 to December 2013, 20 patients with 75 MLM were treated in 34 SRFA sessions with no perioperative mortality. All major complications (n=3) could be treated by the interventional radiologist. On average, two lesions (range, 1–14) per patient with an average size of 1.7 cm (range, 0.5–14.5 cm) were ablated. Four of ten local recurrences were successfully re-treated, whereby a total of 69/75 (92%) lesions were ablated completely. The median, 1-, 3- and 5-year OS rates from the date of the SRFA were 19.3 months, 64%, 41%, and 17, respectively.
SRFA in “difficult” locations and situations
Caudate lobe lesions (26)
After SRFA of 24 caudate lobe HCCs in 20 patients, the local recurrence rate was 4.2% (1/24). The median and 1-, 3-, and 5-year overall survival rates from the date of the first SRFA were 51.3 months, 95%, 59%, and 44%, respectively.
Subcardiac hepatocellular carcinoma (27)
A recent paper demonstrated that SRFA can be applied in tumors adjacent to the heart with satisfactory results. In 79 patients with 114 subcardiac HCCs with a median size of 2.5 cm (range, 0.5–9.5 cm) the local recurrence was 7.0%. The major complication and perioperative mortality rates were 7.7% (8/104) and 1% (1/104), respectively. The 1-, 3- and 5-year OS rates after SRFA of single subcardiac HCCs were 92%, 77%, and 65%, respectively, with a median OS of 90.6 months.
Hepatic dome lesions (28)
In a similar study, the results after SRFA of 238 tumors abutting the diaphragm in the hepatic dome in 177 patients (82 HCCs, 6 ICCs, and 89 metastatic tumors from other origins) were reported. Local tumor recurrence developed in 21 of 238 tumors (8.8%). Twelve (55%) of 22 major complications could be easily treated by the interventional radiologist in the same anesthesia session.
Thermal ablation of CT 'invisible' liver tumors using MRI fusion (17)
In a recent study in 60 patients with 199 lesions not visible in CT underwent SRFA using MRI-fusion and compared to a matched control group without image fusion. In the fusion group, 8.7% of major complications occurred, and the LR rate was 3.5% in HCCs and 4% in metastases. The LR rate of metastasis in the control group was significantly higher, although differences in OS and DFS did not reach statistical significance.
Stereotactic radiofrequency ablation as bail-out strategy for recurrent HCC following hepatic resection (29)
Between 2006 and 2018, 34 consecutive patients with previous HR were treated by SRFA for 140 HCCs, with a median tumor size of 3.0 cm (range, 0.5–10 cm). Local tumor recurrence was observed in 4 of 140 tumors (2.9%). The major complication rate was 4.8% (3 of 60 ablations) with no periprocedural mortality. The reported OS rates at 1-, 3-, and 5-year from the date of the first SRFA were 94.0%, 70.2%, and 53.3%, respectively, with a median OS of 69 months.
SRFA in octogenarians (30)
Thirty-six patients aged older than 80 years underwent SRFA of 70 liver tumors (n=16). Local tumor recurrence was detected in 5 of 70 nodules (7.1%). In 46 SRFA sessions, three major complications (Clavien-Dindo) (6.5%) occurred. The reported overall survival (OS) rates at 1-, 3-, and 5-year from the date of the first SRFA were 84.6%, 50.5%, and 37.9% for HCC patients and 87.5%, 52.5% at 1-, and 3-years for CRC patients.
SRFA of tumors ≥8 cm (31)
Recently, we reported the results of SRFA of 41 primary and secondary liver tumors with a median size of 9.0 cm (range, 8.0–18.0 cm) in 34 consecutive patients with curative intent. Local tumor recurrence (LR) was observed in 4 of 41 tumors (9.8%). The major complication rate and the periprocedural mortality were 20.5% and 2.3%, respectively. Three of nine major complications (pleural effusions) could easily be treated by pleural drainage. The overall survival (OS) rates at 1-, 3-, and 5-year from the date of the first SRFA were 87.5%, 70.0% and 70.0% for patients with intrahepatic cholangiocarcinoma (ICC) and 87.1%, 71.8%, and 62.8% for patients with hepatocellular carcinoma (HCC), respectively. Patients with secondary liver tumors had OS rates of 77.8% and 22.2% at 1- and 3-year.
SRFA in patients with four or more lesions (32)
In another paper, the feasibility, safety, and clinical outcome of simultaneous SRFA of four or more primary and metastatic liver tumors were assessed in a total of 92 patients.
The median sizes of 178 HCCs and 371 liver metastases were 2.2 cm (range, 1.0–8.5 cm) and 3.0 cm (range, 0.5–13 cm), respectively. At initial SRFA, 7 patients (20%) with HCC and 19 (33.3%) with metastases had >6 tumors. Local recurrence was found in 4 of 178 (2.2%) HCCs and in 17 of 371 (4.6%) metastases. Median OS and OS rates at 1, 3, and 5 years were 38.2 months, 88.0%, 54.0%, and 30.4% for patients with HCCs, and 37.4 months, 53.1% and 37.3% for patients with metastases.
Single-probe stereotactic microwave ablation (SMWA)
The Bern group uses a similar device and technique for direct stereotactic placement of microwave probes, without using coaxial needles. The planning, navigation, and image fusion software are comparable to the Treon software. In contrast to our approach, the patient is draped before image acquisition, and sterile reflective skin markers are used for patient tracking. From 2015 to 2017, Tinguely et al. (33) treated 301 primary and secondary liver tumors with a median diameter of 1.5 cm in 191 interventions in 153 patients by stereotactic microwave ablation. The mean targeting positioning error (TPE) per probe was 2.9±2.3 mm. The LR within 6 months was 22% (49 out of 227). 35% of these lesions were successfully re-ablated. Lesion size >30 mm and TPE >5 mm had a significant influence on LR. Challenging lesion locations had no significant influence on targeting accuracy or early ASR.
The same group (34) reported their initial experiences with SMWA for non-colorectal liver metastases (NCRLM) in 23 patients with 40 lesions with a median diameter of 1.4 cm. After a median follow-up of 15 months, the LR rate was 10% (4/40). One patient (4%) had a major complication. The median DFS and the OS were 7 and 18 months, respectively. In another paper (35), they published their experience with SMWA for the treatment of 88 patients with 174 HCCs with a median size of 1.6 cm (range, 0.4–4.5). After a median follow-up of 17.5 months, the LR rate was 6.3%. Proximity to large vessels significantly correlated with LR (P<0.05).
Robot-assisted thermal ablation
So far, only data on accuracy, technical efficacy, and short-term data about percutaneous MWA under robotic guidance is available. Beyer et al. (36) evaluated and compared the needle placement accuracy, patient dose, procedural time, complication rate, and ablation success of MWA using robotic (n=34) and manual guidance (n=30). There was no significant difference between both groups regarding the complication rate and the ablation success. Robotic assistance for liver tumor ablation significantly reduced patient dose and probe positioning time. Also, robotic probe positioning was significantly more accurate (1.6 vs. 3.3 mm, P<0.001). The same group (37) assessed the primary efficacy of robot-assisted MWA and compared it to manually guided MWA for percutaneous ablation of 368 liver tumors in 192 patients (119 ablations with manual guidance, 249 ablations with robotic guidance). The primary technique efficacy outcome of the group treated by robotic guidance was significantly higher than that of the manually guided group (88% vs. 76%; P=0.013). Multiple logistic regression analysis indicated that a small tumor size (≤3 cm) and robotic guidance were significant favorable prognostic factors for complete ablation.
Discussion
After twenty years, we have published studies that show consistently good oncologic outcomes comparable to surgery in terms of local recurrence and overall survival in tumors beyond the current limitations in size and number of lesions for conventional US- and CT-guided thermal ablation. We are still refining the overall methodology to ensure the highest possible reliability of the procedure, like the precise measurement of the ablation margins in the 3D space during the procedure to correct any suboptimal margin. The limitation of this review is that the information is generated from retrospective analyses by a single center, even if it comes from a prospectively made database which includes every patient we treat.
Addressing the question in the title, do we believe that stereotactic thermal ablation will replace minimally invasive surgery for cancer treatment? The short answer is no; ablation will be a great first line option, like minimally invasive surgery is. Ideally, the usage of both minimally invasive approaches—which are to a certain extent complementary and can even be combined in many cases—will decrease our reliance on open surgery. It is worth mentioning, that the use of ablation for treatment of the primary tumor doesn’t preclude the lymphadenectomy—minimally invasive or open—whenever it adds value for either staging or in the oncologic outcome. The point is having an arsenal of treatments to always offer the best possible option for the specific disease and underlying conditions of the patient.
Before getting there, there are multiple things to solve regarding ablation.
First, the methodology has to become approachable consistently by more than one institution treating liver cancer. Despite the consistently good results, this technique is still used only in our center, and stereotactic approaches, in general, are used in very few centers worldwide.
The reasons are manifold. Most hospitals would require additional investments to have a dedicated CT for stereotactic interventions (or at least enough CT room time), a 3D navigation system and availability of general anesthesia. Also, training of a dedicated team, including the interventional oncologist, radiation technician, and anesthetist is necessary.
The reimbursement of percutaneous thermal ablation is still low as compared to the surgical resection and even surgical ablation (38). Therefore, it is difficult to convince the hospital administration to invest in this field.
This technique can be learned in a few months of mentoring (21). Still, the planning itself is done with the patient already under general anesthesia and takes between 5–60 minutes depending on complexity.
A parallel that could be made is the added complexity of laparoscopic liver surgery compared to open surgery. Even with a better reimbursement than open surgery and clear advantages, it still is not done in the majority of cases, except in a handful of centers around the world (39-41).
This makes it understandable that most interventional radiologists do not see an argument to invest time and money in establishing a sophisticated technique, changing their existing CT- or US-guided “freehand” guidance technique. Particularly given that reimbursement for percutaneous ablation is minimal. This situation is much better for surgeons, who are reimbursed for ablation a similar amount to resection, with lower costs (42).
However, if thermal ablation can provide oncologic outcomes comparable to resection consistently, the decision of what to do will depend on many factors. Non-modifiable is tumor location, were subcapsular tumors are relatively simple to resect and relatively complex to ablate (43). Also, tumors close to bile ducts are not optimal for ablation, even if their surgical resection—particularly minimally invasive tissue-sparing resection—is challenging too. Local expertise, technical capability, and reimbursement are also factors, but patient preference has always been the most crucial driver. Given technical feasibility and similar expected outcomes, patients tend to prefer the least invasive option (44,45). Percutaneous cardiac revascularization, laparoscopic cholecystectomy, and ESD are great examples. Liver ablation will likely be the same, and the effect of this will be that an increasing proportion of patients will be treated this way.
Non-surgical treatments with curative intent already outnumber surgery in the treatment of HCC in Japan (46). A French multicentric study (47) showed that increasing the surveillance in patients at risk, led 70% of patients being treated curatively, half of them with ablation. In line with this, the Swedish database (SweLiv) shows ablation as a curative therapy for HCC in 60% of cases.
The advancement of ablation and minimally invasive resections will bring challenges; the teams of people treating liver cancer will have to have a higher integration and possibly cross-training, even if the multiple available options are hard to master for any individual.
Improvements in technology, like digital assistance for the procedures, robotics, and ultimately, automation, will eventually solve these challenges. Nonetheless, the transition to that stage requires that we keep working to improve training, simplify techniques, bring down the barriers between specialties, and to improve the gathering of clinical evidence.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Robert Sucher and Elisabeth Sucher) for the series “Minimally Invasive Liver Surgery” published in Laparoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/ls-20-107
Peer Review File: Available at http://dx.doi.org/10.21037/ls-20-107
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-107). The series “Minimally Invasive Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. RB reports personal fees from CAScination, during the conduct of the study; personal fees from CAScination, outside the submitted work. AA reports working for Siemens Healthineers to help develop the field of image-guided interventions. Neither his salary nor incentives is related in any way to the information presented in this publication. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Laimer G, Schullian P, Jaschke N, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol 2020;30:2463-72. [Crossref] [PubMed]
- Kim YS, Rhim H, Lim HK, et al. Completeness of treatment in hepatocellular carcinomas treated with image-guided tumor therapies: Evaluation of positive predictive value of contrast-enhanced CT with histopathologic correlation in the explanted liver specimen. J Comput Assist Tomogr 2006;30:578-82. [Crossref] [PubMed]
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology sans frontieres meeting 2013. Eur Radiol 2015;25:3438-54. [Crossref] [PubMed]
- Berber E. The first clinical application of planning software for laparoscopic microwave thermosphere ablation of malignant liver tumours. HPB (Oxford) 2015;17:632-6. [Crossref] [PubMed]
- Dodd GD 3rd, Frank MS, Aribandi M, et al. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol 2001;177:777-82. [Crossref] [PubMed]
- Widmann G, Schullian P, Haidu M, et al. Respiratory motion control for stereotactic and robotic liver interventions. Int J Med Robot 2010;6:343-9. [Crossref] [PubMed]
- Engstrand J, Toporek G, Harbut P, et al. Stereotactic CT-Guided Percutaneous Microwave Ablation of Liver Tumors With the Use of High-Frequency Jet Ventilation: An Accuracy and Procedural Safety Study. AJR Am J Roentgenol 2017;208:193-200. [Crossref] [PubMed]
- Galmén K, Jakobsson JG, Freedman J, et al. High Frequency Jet Ventilation during stereotactic ablation of liver tumours: an observational study on blood gas analysis as a measure of lung function during general anaesthesia. F1000Res 2019;8:386. [Crossref] [PubMed]
- Lee SJ, Quek KH. Facilitating Airway Surgery in a Morbidly Obese Patient Using Transnasal Humidified Rapid Insufflation Ventilatory Exchange (THRIVE). Case Rep Anesthesiol 2018;2018:5310342 [Crossref] [PubMed]
- To K, Harding F, Scott M, et al. The use of Transnasal Humidified Rapid-Insufflation Ventilatory Exchange in 17 cases of subglottic stenosis. Clin Otolaryngol 2017;42:1407-10. [Crossref] [PubMed]
- Bale R, Freund M, Bodner G, et al. Precise Computer- assisted Liver Tumor Puncture for Biopsy and Thermal Ablation. Radiology 2002;225:242.
- Bale RJ, Laimer I, Martin A, et al. Frameless stereotactic cannulation of the foramen ovale for ablative treatment of trigeminal neuralgia. Neurosurgery 2006;59:ONS394-401; discussion ONS402. [PubMed]
- Bale R, Widmann G, Haidu M. Stereotactic radiofrequency ablation. Cardiovasc Intervent Radiol 2011;34:852-6. [Crossref] [PubMed]
- Bale R, Widmann G, Schullian P, et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol 2012;22:930-7. [Crossref] [PubMed]
- Schullian P, Johnston E, Laimer G, et al. Thermal ablation of CT 'invisible' liver tumors using MRI fusion: a case control study. Int J Hyperthermia 2020;37:564-72. [Crossref] [PubMed]
- Ishizaka H, Awata S, Arai H, et al. CT-guided radiofrequency liver tumour ablation: use of a two-step coaxial system with a fine guide needle wire unit for high-risk cases. Br J Radiol 2010;83:1077-9. [Crossref] [PubMed]
- Hetzer B, Vogel GF, Entenmann A, et al. Stereotactic radiofrequency ablation of a variety of liver masses in children. Int J Hyperthermia 2020;37:1074-81. [Crossref] [PubMed]
- Widmann G, Schullian P, Haidu M, et al. Targeting accuracy of CT-guided stereotaxy for radiofrequency ablation of liver tumours. Minim Invasive Ther Allied Technol 2011;20:218-25. [Crossref] [PubMed]
- Widmann G, Schullian P, Haidu M, et al. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol 2012;35:570-80. [Crossref] [PubMed]
- Bale R, Schullian P, Eberle G, et al. Stereotactic Radiofrequency Ablation of Hepatocellular Carcinoma: a Histopathological Study in Explanted Livers. Hepatology 2019;70:840-50. [Crossref] [PubMed]
- Haidu M, Dobrozemsky G, Schullian P, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol 2012;35:1074-82. [Crossref] [PubMed]
- Bale R, Richter M, Dunser M, et al. Stereotactic Radiofrequency Ablation for Breast Cancer Liver Metastases. J Vasc Interv Radiol 2018;29:262-7. [Crossref] [PubMed]
- Bale R, Schullian P, Schmuth M, et al. Stereotactic Radiofrequency Ablation for Metastatic Melanoma to the Liver. Cardiovasc Intervent Radiol 2016;39:1128-35. [Crossref] [PubMed]
- Schullian P, Laimer G, Putzer D, et al. Stereotactic radiofrequency ablation of primary liver tumors in the caudate lobe. HPB (Oxford) 2020;22:470-8. [Crossref] [PubMed]
- Schullian P, Johnston EW, Putzer D, et al. Stereotactic radiofrequency ablation of subcardiac hepatocellular carcinoma: a case-control study. Int J Hyperthermia 2019;36:876-85. [Crossref] [PubMed]
- Schullian P, Putzer D, Laimer G, et al. Feasibility, safety, and long-term efficacy of stereotactic radiofrequency ablation for tumors adjacent to the diaphragm in the hepatic dome: a case-control study. Eur Radiol 2020;30:950-60. [Crossref] [PubMed]
- Schullian P, Laimer G, Putzer D, et al. Stereotactic radiofrequency ablation as first-line treatment of recurrent HCC following hepatic resection. Eur J Surg Oncol 2020;46:1503-9. [Crossref] [PubMed]
- Schullian P, Putzer D, Silva MA, et al. Stereotactic Radiofrequency Ablation of Liver Tumors in Octogenarians. Front Oncol 2019;9:929. [Crossref] [PubMed]
- Schullian P, Johnston EW, Putzer D, et al. Safety and efficacy of stereotactic radiofrequency ablation for very large (≥8 cm) primary and metastatic liver tumors. Sci Rep 2020;10:1618. [Crossref] [PubMed]
- Schullian P, Putzer D, Eberle G, et al. Simultaneous Stereotactic Radiofrequency Ablation of Multiple (≥ 4) Liver Tumors: Feasibility, Safety, and Efficacy. J Vasc Interv Radiol 2020;31:943-52. [Crossref] [PubMed]
- Tinguely P, Frehner L, Lachenmayer A, et al. Stereotactic Image-Guided Microwave Ablation for Malignant Liver Tumors-A Multivariable Accuracy and Efficacy Analysis. Front Oncol 2020;10:842. [Crossref] [PubMed]
- Perrodin S, Lachenmayer A, Maurer M, et al. Percutaneous stereotactic image-guided microwave ablation for malignant liver lesions. Sci Rep 2019;9:13836. [Crossref] [PubMed]
- Lachenmayer A, Tinguely P, Maurer MH, et al. Stereotactic image-guided microwave ablation of hepatocellular carcinoma using a computer-assisted navigation system. Liver Int 2019;39:1975-85. [Crossref] [PubMed]
- Beyer LP, Pregler B, Niessen C, et al. Robot-assisted microwave thermoablation of liver tumors: a single-center experience. Int J Comput Assist Radiol Surg 2016;11:253-9. [Crossref] [PubMed]
- Schaible J, Pregler B, Verloh N, et al. Improvement of the primary efficacy of microwave ablation of malignant liver tumors by using a robotic navigation system. Radiol Oncol 2020;54:295-300. [Crossref] [PubMed]
- Loveman E, Jones J, Clegg AJ, et al. The clinical effectiveness and cost-effectiveness of ablative therapies in the management of liver metastases: systematic review and economic evaluation. Health Technol Assess 2014;18:vii-viii, 1-283. [Crossref] [PubMed]
- Fretland AA, Dagenborg VJ, Bjornelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2014;21:723-31. [Crossref] [PubMed]
- Brough D, O’Rourke N. Laparoscopic hepatic resection. Laparosc Surg 2020;4:18. [Crossref]
- Takahashi H, Akyuz M, Kahramangil B, et al. A Comparison of the Initial Cost Associated With Resection Versus Laparoscopic Radiofrequency Ablation of Small Solitary Colorectal Liver Metastasis. Surg Laparosc Endosc Percutan Tech 2018;28:371-4. [Crossref] [PubMed]
- Cho JY, Han HS, Wakabayashi G, et al. Practical guidelines for performing laparoscopic liver resection based on the second international laparoscopic liver consensus conference. Surg Oncol 2018;27:A5-9. [Crossref] [PubMed]
- Plaisier PW, Berger MY, van der Hul RL, et al. Unexpected difficulties in randomizing patients in a surgical trial: a prospective study comparing extracorporeal shock wave lithotripsy with open cholecystectomy. World J Surg 1994;18:769-72; discussion 773. [Crossref] [PubMed]
- Varadarajulu S, Tamhane A, Drelichman ER. Patient perception of natural orifice transluminal endoscopic surgery as a technique for cholecystectomy. Gastrointest Endosc 2008;67:854-60. [Crossref] [PubMed]
- Kudo M. Management of Hepatocellular Carcinoma in Japan as a World-Leading Model. Liver Cancer 2018;7:134-47. [Crossref] [PubMed]
- Trinchet JC, Bourcier V, Chaffaut C, et al. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort). Hepatology 2015;62:737-50. [Crossref] [PubMed]
Cite this article as: Bale R, Schullian P, Alzaga A. Narrative review of 3D navigated stereotactic liver ablation—do we still need a minimally invasive liver surgeon? Laparosc Surg 2021;5:35.


