
Analysis of open and laparoscopic liver resections in a German high-volume liver tumor center
Introduction
Minimally invasive liver surgery is a maturing field (1). Since the initial report in 1992 by Gagner et al. (2) the incidence of laparoscopic liver resections (LLR) has increased exponentially (3). Initial concerns with regard to oncological inferiority and technical inapplicability have been scientifically disproved and even outplayed by reduced morbidity, and mortality in selected groups of patients (4).
“First movers” in this novel field of minimally invasive liver surgery comprised groups in Asia, the United States and Europe. Consensus guidelines have been established (5) and constantly updated (6) and adapted to anticipated new challenges (7). Today, difficulty scoring methodologies help to estimate and rank the complexity of a minimally invasive liver resection (8). However, open and minimally invasive resections cannot be compared one to one. In contrast to open procedures, which are graded in major and minor, depending on the amount of liver segments resected (according to the Brisbane 2000 terminology) (9), minimally invasive resections of posterosuperior segments, which are difficult to access with conventional laparoscopic instruments, justify for a “technical major” designation (10), which would be classified as minor according to the conventional open surgical terminology.
In recent years there has been a significant increase in the number of series published on LLR, that include single center series. In Germany, our team was among the first to adapt to laparoscopic liver surgery. Initially we shared the opinion that LLR was ideally suited for the resection of Hepatocellular carcinoma (HCC) in patients with cirrhosis (11,12). With an increase in experience and the implementation of open surgical strategies into the minimally invasive liver surgical world, more complex procedures were accomplished to the patients benefits owing to our increasing capability (13). Among other things, this included the implementation of novel intraoperative visualization techniques like hyperspectral imaging (14) and indocyanine green (ICG) staining (15,16). Today laparoscopic hemi-hepatectomies are performed on a regular basis for both malignant and benign indications and even extended resections for perihilar cholangiocarcinoma (pCCA) requiring biliary reconstruction are commonly addressed laparoscopically in specialized liver tumor centers (17). In this study, we aimed to analyze perioperative and short-term postoperative outcomes of our patients requiring liver resection for benign and malignant disease. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ls-20-93).
Methods
Our prospectively maintained Liver Tumor Center database for patients undergoing liver resection was analyzed for the years 2018 and 2019. This study was conducted in congruence with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of the University Clinic of Leipzig (Ref. #: 142/18-ek). Because of the retrospective nature of the research, the requirement for informed consent was waived. The primary outcome measure was short-term surgical outcome. All liver resections were performed or assisted by the same two surgeons.
Before surgery, each case was reviewed in a multidisciplinary tumor-board meeting. In principle every patient was primarily evaluated for LLR. This included patients with resectable liver disease, independent of the liver segment affected, and sufficient functional parenchyma and liver function, which was measured by CT volumetry and the LiMAx test (18). Patients with tumor disease of the liver hilus, involving central vascular structures requiring vascular reconstruction during resection, and patients receiving portal vein embolization for liver augmentation, were not considered for LLR at that time.
Patient demographics, pathologic diagnosis, radiologic findings, and peri- and intraoperative surgical data were reviewed. The extent of OLR was graded according to the Brisbane 2000 terminology of liver anatomy and resections (9). LLRs were classified according to both the Asian (8,19) and European (10) difficulty scoring systems. The Clavien-Dindo classification was used for morbidity assessment, and major morbidity was defined as being Clavien Dindo 3b or greater (20).
Surgical technique
Open liver resections (OLR) were performed as described earlier by our group (14,21,22). For laparoscopic resections we preferred a supine patient position with split legs, with the surgeon standing between the legs and the assistant on the left side of the patient. Intraoperative ICG counter perfusion staining was utilized in anatomic liver resections following inflow control and direct ICG tumor staining was employed for intraoperative tumor demarcation of HCC, CCA and CRLM (15). If appropriate, a laparoscopic liver hanging maneuver was utilized for extensive resections to reduce bleeding during the parenchymal phase and furthermore simplify the procedure (13). A tourniquet around the hepatoduodenal ligament was always placed prior to resection, to facilitate a Pringle Maneuver in case of bleeding.
Special laparoscopic instruments comprised ultrasonic shears (Harmonic, Ethicon®) a laparoscopic CUSA (Caviton Ultrasonic Surgical Aspirator), and bipolar forceps. Laparoscopic ultrasound was performed for intraoperative tumor visualization and vascular anatomy mapping in every case. All patients undergoing OLR and LLR received overnight intensive care and were discharged to normal care earliest on postoperative day one.
Statistical analysis
Data was collected retrospectively and a database of previously determined variables was generated. A t-test was used to determine statistical significance. A P value <0.05 was considered as statistically relevant. Statistical analyses were performed using Microsoft excel 2018.
Results
In the time period investigated, a total number of n=231 patients received a liver resection in our institution. An early termination of the operation without resection was necessary in n=14 (7%) of cases due to histologically confirmed peritoneal metastasis which was not detected in the staging CT or MRI and therefore subsequently excluded from the final study population (n=217). Patient demographics and most frequent pathologic diagnoses are displayed in Table 1.

Full table
In short, n=124 (57%) patients received OLR and n=93 (43%) was operated with a minimally invasive approach (LLR). From all minimally invasive treated patients, n=73 (79%) received a totally laparoscopic operation and n=15 (16%) patients were operated in a laparoscopic-hand-assisted manner. This exclusively applied to resections of the posterosuperior segments 7, 8 and 4a. In n=5 cases (5%) a conversion to open surgery was necessary due to laparoscopic hand-assisted inaccessibility n=4 (80%), or tumor infiltration into other organs (diaphragm, inferior vena cava) in combination with morbid obesity [n=1, (20%)].
Mean patient age was significantly higher in the OLR group (65±12 years), when compared to the LLR group (LLR: 59±14 years; P=0.002), and the sex ratio (female/male) was in favor of men, in both groups [OLR: f:m=54% (n=51):59% (n=73) vs. LLR: f:m=41% (n=43):46% (n=50)], respectively.
A total number of n=166 patients (77%) were operated for malignant disease and n=51 patients (24%) were operated for benign indications. This larger number of oncologic operations remained valid for both open [OLR for malignant indication: n=109 (88%) vs. OLR for benign indications: n=15 (12%)] and laparoscopic resections [LLR for malignant indication: n=57 (61%) vs. LLR for benign indications: n=36 (39%)].
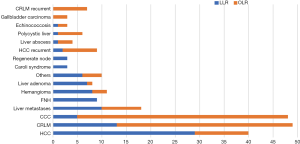
Patients with Cholangiocarcinoma (CCA) and Colorectal Liver Metastases (CRLM) were predominantly treated by OLR [OLR for CCA: n=42 (34%) vs. LLR for CCA: n=4 (4%) and OLR for CRLM: n=42 (34%) vs. LLR for CRLM: n=12 (13%)], whereas patients with HCC to a greater extent received a LLR [LLR for HCC: n=30 (32%) vs. OLR for HCC: n=18 (15%)]. The three major benign indications for liver resection comprised: giant hemangioma [n=11 (5%)], symptomatic focal nodular hyperplasia (FNH) [n=9 (4%)] and liver adenoma [n=8 (4%)]. A total number of n=61 (28%) of patients had previous upper abdominal surgery [OLR with prior abdominal surgery n=45 (36%) and LLR with prior abdominal surgery n=16 (19%)]. This included previous liver resections [n=31 (14%)], cholecystectomies [n=20 (9%)], gastric resections [n=7 (3%)], splenectomies [n=2 (1%)] and prior liver transplantation [n=1 (1%)] etc. A detailed distribution of OLR and LLR with regard to the underlying diagnosis is displayed in Figure 1.
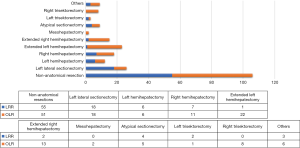
With regard to the extent of liver surgery, non-anatomical resections [n=101 (47%)] were the most frequent operations in our cohort with n=51 (55%) performed by LLR, and n=50 (40%) performed by OLR. With a total of n=28 (13%) anatomic right and left hemihepatectomies were the second most common surgical procedures, both commonly performed by LLR [left hemihepatectomy LLR: n=6 (7%), vs. left hemihepatectomc OLR: n=6 (5%) and right hemihepatectomy LLR: n=7 (8%), vs. right hemihepatectomc OLR: n=9 (7%)]. Left lateral (Segment 1 and 2) resections n=26 (12%), were the third most common resections in our cohort, predominantly performed by LLR [left lateral LLR: n=18 (19%) vs. left lateral OLR: n=8 (7%)]. In n=33 (15%) cases an extended resection was necessary, which was predominantly performed by OLR [LLR: n=3 (3%), OLR: n=30 (24%)]. Two out of 11 trisectionectomies were performed by LLR [Trisectionectomy LLR: 2 (2%) vs. Trisectionectomy OLR: 9 (7%)]. Two mesohepatectomies (2%) were performed by open surgery. Laparoscopic lymphadenectomy was performed in patients with CCC and one patient with HCC. A detailed description of types of liver resections performed is given in Figure 2.
Surgical data
The mean operative time was longer for OLR with 341 min (range, 141–556 min) when compared to LLR [273 min (range, 44–590 min), (P<0.001)] and intraoperative blood transfusions were necessary in n=7 (3%) cases [intraoperative transfusion LLR: n=3 (3%) vs. intraoperative transfusion OLR: n=4 (3%)]. Mean length of hospital stay was 14±13 days (LLR: 9±6 vs. OLR: 18±15 days; P<0.001). Abdominal drains were placed in 41% (n=88) of cases [abdominal drain LLR: n=21 (22%) vs. abdominal drain OLR n=67 (54%)]. Intraoperative biliary drainages [n=102 (47%)] were predominantly placed in OLR cases [n=95 (77%)] when compared to LLR cases [n=7 (8%)]. Radical Lymphadenectomy was performed in n=74 cases (34%); [radical lymphadenectomy LLR: n=6 (7%) vs. radical lymphadenectomy OLR: n=68 (55%)]. R0 resection was achieved in n=197 (91%) of cases [R0 resection LLR: n=90 (98%) vs. R0 resection OLR: n=107 (86%)]. R0 resection rates were highest for CRLM resections [LLR: n=12 (100%) vs. OLR: n=38 (91%)] followed by HCC resections [LLR: n=27 (90%) vs. OLR: n=16 (89%)] and CCA resections [LLR: n=3 (75%) vs. OLR: n=31 (74%)]. A detailed description of surgical results is provided in Table 2.

Full table
Difficulty scoring and morbidity and mortality outcome
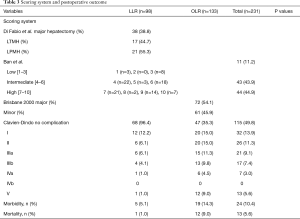
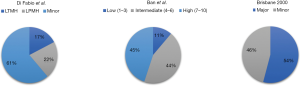
A detailed description of classifications and difficulty scoring of liver resections and complications after liver resection for our patient group is given in Table 3. In short, according to the Brisbane 2000 terminology of liver anatomy and resections, n=64 (52%) patients were treated by major and n=60 (48%) by minor resections.
Full table
According to Di Fabio et al. out of n=93 LLRs, n=36 (39%) was classified as laparoscopic major hepatectomies comprising n=16 (44%) traditional major laparoscopic hepatectomies (LTMH) and n=20 (56%) laparoscopic posterosuperior major hepatectomies (LPMH), which were technically challenging as they are considered difficult to approach using straight laparoscopic instruments.
According to Ban et al. out of the n=93 minimally invasive operated LLRs, n=7 (8%) was of low, n=42 (45%) was of intermediate and n=44 (47%) was of high difficulty, with regard to performance (Figure 3).
Major morbidity, defined as Clavien Dindo 3b or greater was 11% (n=23). Patients with LLR had a significantly lower morbidity [morbidity rate LLR: n=5 (5%)] when compared to the OLR group [morbidity rate OLR: n=18 (15%)]. Likewise, overall in-hospital mortality n=13 (6%) was very low in the LLR group [in hospital mortality LLR: n=1 (1%)] when compared to OLR group [in hospital mortality OLR: n=12 (10%)].
Discussion
Our data demonstrate that despite high numbers of complex liver resections, morbidity and mortality rates were low in our analyzed patient group. The fact that a fraction of more than 42% of all liver resections were performed minimally invasive in the years 2018 and 2019, furthermore reflects our key of propelling minimal invasive surgical techniques for the liver.
The majority (77%) of our patients received an oncologic resection. This was true for both OLR and LLR and resembles the global attitude that minimally invasive techniques are not reserved for selected tumor entities. However, in parallel we would like to strike that the indication for a liver resection must not be loosened especially for benign indications just because of minimal invasive accessibility (23).
Patients with HCC in cirrhosis accounted for the major part of LLRs in our collective. Analysis of the literature confirms that minor liver resections for HCC even in cirrhotic livers should be the approach of first choice (24). This might especially be true for lesions less than 5 cm in diameter (25). A recent propensity score matched analysis demonstrated that in terms of oncologic outcome and surgical outcome, a selected group of patients even might benefit from major LLR for HCC in cirrhosis (26). Decades ago, it has been shown that liver resection prior to transplantation did not increase the morbidity or impair long-term survival following liver transplantation (27). Recent work indicated that salvage liver transplantation after laparoscopic resection for HCC was even more feasible and save, achieving excellent short-term results (28). A recent meta-analysis demonstrated that surgical resection also achieved a better overall-survival when compared to Trans Arterial Chemo Embolization (TACE) in patients with HCC. Therefor laparoscopic resections might be able to partially replace TACE as a bridging therapy to liver transplantation. However, it is clear that efficient bridging strategies for patients with HCC are even more important (29) especially in countries like Germany where waiting time for liver transplantation is long.
Few patients diagnosed with CCA received LLR in our cohort. In this patient collective a minimally invasive approach was predominantly considered for intrahepatic Cholangiocarcinomas (iCCA), which did not require extrahepatic bile duct resection. A recent meta-analysis confirmed that LLR for iCCA achieved excellent surgical outcomes and provided short-term benefits over OLR without negatively affecting oncologic adequacy in terms of R0 resections and disease recurrence (30). Our patient collective only comprised one case with perihilar Cholangiocarcinoma (pCCA) which required Roux – Y laparoscopic bile-duct reconstruction. Similar cases have been published as case reports recently (31). Surgical resection still represents the mainstay of pCCA treatment (32). However, if laparoscopic surgery is capable to replace our initially postulated open surgical resection strategy for pCCA (21) requires further investigations.
Radical lymphadenectomy is mandatory in patients with CCA. Recent data indicate that the laparoscopic technique does not compromise accuracy and outcome of nodal dissection (33,34). From a technical perspective, delicate vascular reconstruction after portal vein resection and biliary reconstruction, represents the “Achilles heel” of a pCCA resection. Performance of vascular and biliary reconstruction with laparoscopic instruments is even more challenging. To overcome the hurdle of restricted visibility and maneuverability we recently introduced a parachute suturing technique for biliary reconstruction in patients receiving a laparoscopic pCCA resection (35). This technique provided a superior view on the anastomosis and facilitated an unrestrained completion of the anastomosis. Although robotic surgery is supposed to deliver substantial benefits over laparoscopic surgery especially when it comes to delicate vascular reconstruction, first data do not support its continued practice on pCCA cases until significant technical and instrumental refinements become available (36).
The liver is the most common site of metastasis in patients with colorectal cancer. In Europe the overall liver metastasis rate from colorectal cancer has been reported to be up to 23% (37). Surgical resection is currently still the only curative treatment modality. The OSLO-COMET randomized controlled trial demonstrated that in patients undergoing parenchyma-sparing liver resection for colorectal metastases, laparoscopic surgery was associated with significantly less postoperative complications when compared to open surgery (38). Patients with CRLM represent the centerpiece of our study population. However, only a fraction of 13% was treated by LLR. High tumor load requiring future liver remnant augmentation strategies (39) were the main reason for the necessity of an OLR strategy. Up to 14% of CRLM may be synchronously detected (40). As described by other groups, in case of synchronous liver metastases, we favored a simultaneous laparoscopic resection (together) rolled into one with primary tumor resection. Provided that the extent of liver resection required was minor (41), including wedge resections, single segmentectomies or left lateral resections. In case of higher tumor loads chemotherapy was administered prior to major liver resection (42).
The basis of a curative liver resection is built on negative resection margins. Overall R0 resection rate was 91%. Our data show that LLR achieved better R0 resection rates than OLR, however this was not a case matched study, and a direct comparison is hence invalid. Nevertheless, our data demonstrate that at least the introduction of LLRs into our program did not impair R0 resection rates. The margin status remains a very important factor in hepatectomies independent of the tumor entity.
Benign liver tumors represent a challenge in clinical management and there is considerable controversy with respect to the indications for surgery as the evidence for surgical treatment is variable (43). Recent data indicate that patients with preoperative symptoms from adenoma and focal nodular hyperplasia (FNH) show a high rate of postoperative symptom relieve (44). From a global perspective, most initial minimally invasive liver resections were typically done for benign lesions in anterior or left lateral segments (45). In our patient group the majority of benign lesions was operated by LLR.
Difficulty scoring and correct taxonomy for liver resections is vital for the establishment and maintenance of an academic liver surgery program. It is not only key for the scientific evaluation of patient data and quality assessment but also helps trainees in their buildup of surgical skills. Especially in the field of minimally invasive surgery difficulty scoring is required to guide surgeons in advancing from simple to difficult resections. We applied the two most common difficulty scoring systems used in Asia and Europe to our patient cohort. Ban et al. provided a scoring system based on preoperative parameters which comprise the extent of liver resection, tumor location, tumor size, liver function, and tumor proximity to major vessels (8,19). Accordingly, difficulty of laparoscopic resections can be graded as low, intermediate and high. Di Fabio et al. highlighted the fact that liver resections of segments from the posterosuperior segments may be graded as technically major if performed laparoscopically due to the difficult laparoscopic accessibility of these segments. A recent landmark paper by Filmann et al. revealed that overall hospital mortality after liver resection is high in Germany (46). With an overall hospital mortality rate of 5.9% for our cohort we were able to achieve good results. Especially the low mortality rate of 1% in the LLR group confirms that our development of a minimally invasive liver resection program should be on the right track.
The learning curve in laparoscopic liver surgery consists of different phases in which hepatobiliary surgeons stepwise edge through more and more complex cases (47). We started our program with smaller resections of left lateral and anteromedial segments predominantly in patients with HCC in liver cirrhosis (12). Little by little we gained more confidence in doing anatomic hemihepatectomies and even extended liver resections (13). We share the opinion that major hepatectomies might have a learning curve of 45–60 cases (48). With a case load of 60–100 LLRs per year we are well aware that it takes time to accomplish individual goals. In our unit all laparoscopic resections were performed by the same surgical team. With an experience of more than 7 years we currently aim to establish a training program for fellows interested in minimally invasive liver surgery. In this context we share the opinion that inter-institutional collaboration and exchange of skills might enable a synergistic development of techniques for safe progression to more complex surgeries (49). The fact that we are still operating on a highly selected patient collective however makes a general comparison to open liver surgery cases difficult.
Acknowledgments
Funding: We acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ls-20-93
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ls-20-93
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-93). Robert Sucher serves as an unpaid editorial board member of Laparoscopic Surgery. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in congruence with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of the University Clinic of Leipzig (Ref. #: 142/18-ek). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sucher E, Sucher R. Minimally invasive liver surgery: a field is maturing. Laparosc Surg 2019;3:34. [Crossref]
- Gagner M, Rogula T, Selzer D. Laparoscopic liver resection: benefits and controversies. Surg Clin North Am 2004;84:451-62. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Gavriilidis P, Roberts KJ, Aldrighetti L, et al. A comparison between robotic, laparoscopic and open hepatectomy: A systematic review and network meta-analysis. Eur J Surg Oncol 2020;46:1214-24. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Ban D, Kudo A, Ito H, et al. The difficulty of laparoscopic liver resection. Updates Surg 2015;67:123-8. [Crossref] [PubMed]
- Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351-5. [Crossref] [PubMed]
- Di Fabio F, Samim M, Di Gioia P, et al. Laparoscopic major hepatectomies: clinical outcomes and classification. World J Surg 2014;38:3169-74. [Crossref] [PubMed]
- Seehofer D, Sucher R, Schmelzle M, et al. Evolution of laparoscopic liver surgery as standard procedure for HCC in cirrhosis? Z Gastroenterol 2017;55:453-60. [Crossref] [PubMed]
- Seehofer D, Sucher R. Long term outcome of laparoscopic liver resection for HCC—is the jury still out? Laparosc Surg 2018;2:72. [Crossref]
- Sucher R, Hau HM, Rademacher S, et al. Totally Minimally Invasive Extended Right Hepatectomy Using the Intracorporal Liver Hanging Maneuver. Videoscopy 2019;29.
- Sucher R, Athanasios A, Kohler H, et al. Hyperspectral Imaging (HSI) in anatomic left liver resection. Int J Surg Case Rep 2019;62:108-11. [Crossref] [PubMed]
- Sucher R, Rademacher S, Lederer A, et al. Laparoscopic Left Hemihepatectoy Applying Intraoperative Indocyanine Green Fluorescence Detection Counter Perfusion Method for Visualization. Zentralbl Chir 2020;145:135-7. [PubMed]
- Sucher R, Brunotte M, Seehofer D. Indocyanine green fluorescence staining in liver surgery. Chirurg 2020;91:466-73. [Crossref] [PubMed]
- Sucher R, Scheuermann U, Seehofer D. Total Laparoscopic Resection of Hilar Cholangiocarcinoma type IIIb Using a Parachute Technique for Hepaticojejunostomy. Ann Surg Oncol 2020. [Epub ahead of print]. [Crossref]
- Stockmann M, Lock JF, Malinowski M, et al. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford) 2010;12:139-46. [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg 1999;230:808-18; discussion 19. [Crossref] [PubMed]
- Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol 2012;19:1602-8. [Crossref] [PubMed]
- Farges O, Goutte N, Dokmak S, et al. How surgical technology translates into practice: the model of laparoscopic liver resections performed in France. Ann Surg 2014;260:916-21; discussion 21-2. [Crossref] [PubMed]
- Fuks D, Aldrighetti L, Jiao LR, et al. Laparoscopic Management of Hepatocellular Carcinoma: A Critical Reappraisal. Surg Laparosc Endosc Percutan Tech 2017;27:203-5. [Crossref] [PubMed]
- Yoon SY, Kim KH, Jung DH, et al. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc 2015;29:2628-34. [Crossref] [PubMed]
- Yoon YI, Kim KH, Kang SH, et al. Pure Laparoscopic Versus Open Right Hepatectomy for Hepatocellular Carcinoma in Patients With Cirrhosis: A Propensity Score Matched Analysis. Ann Surg 2017;265:856-63. [Crossref] [PubMed]
- Belghiti J, Cortes A, Abdalla EK, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg 2003;238:885-92; discussion 92-3. [Crossref] [PubMed]
- Felli E, Cillo U, Pinna AD, et al. Salvage liver transplantation after laparoscopic resection for hepatocellular carcinoma: a multicenter experience. Updates Surg 2015;67:215-22. [Crossref] [PubMed]
- Belghiti J, Carr BI, Greig PD, et al. Treatment before liver transplantation for HCC. Ann Surg Oncol 2008;15:993-1000. [Crossref] [PubMed]
- Guerrini GP, Esposito G, Tarantino G, et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: the first meta-analysis. Langenbecks Arch Surg 2020;405:265-75. [Crossref] [PubMed]
- Zhang CW, Liu J, Hong DF, et al. Pure laparoscopic radical resection for type IIIa hilar cholangiocarcinoma. Surg Endosc 2018;32:1581-2. [Crossref] [PubMed]
- Ito F, Cho CS, Rikkers LF, et al. Hilar cholangiocarcinoma: current management. Ann Surg 2009;250:210-8. [Crossref] [PubMed]
- Ratti F, Fiorentini G, Cipriani F, et al. Perioperative and Long-Term Outcomes of Laparoscopic Versus Open Lymphadenectomy for Biliary Tumors: A Propensity-Score-Based, Case-Matched Analysis. Ann Surg Oncol 2019;26:564-75. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma: a propensity score-based case-matched analysis from a single institution. Surg Endosc 2016;30:1999-2010. [Crossref] [PubMed]
- Sucher R, Seehofer D. Hepatobiliary Surgeons are Spurred to Implement Totally Minimally Invasive Techniques for Perihilar Cholangiocarcinoma Surgery. Ann Surg Oncol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Xu Y, Wang H, Ji W, et al. Robotic radical resection for hilar cholangiocarcinoma: perioperative and long-term outcomes of an initial series. Surg Endosc 2016;30:3060-70. [Crossref] [PubMed]
- Gatta G, Capocaccia R, Sant M, et al. Understanding variations in survival for colorectal cancer in Europe: a EUROCARE high resolution study. Gut 2000;47:533-8. [Crossref] [PubMed]
- Fretland AA, Dagenborg VJ, Bjornelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Neumann UP, Seehofer D, Neuhaus P. The surgical treatment of hepatic metastases in colorectal carcinoma. Dtsch Arztebl Int 2010;107:335-42. [Crossref] [PubMed]
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [Crossref] [PubMed]
- Bizzoca C, Delvecchio A, Fedele S, et al. Simultaneous Colon and Liver Laparoscopic Resection for Colorectal Cancer with Synchronous Liver Metastases: A Single Center Experience. J Laparoendosc Adv Surg Tech A 2019;29:934-42. [Crossref] [PubMed]
- Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 2006;243:1-7. [Crossref] [PubMed]
- Hoffmann K, Unsinn M, Hinz U, et al. Outcome after a liver resection of benign lesions. HPB (Oxford) 2015;17:994-1000. [Crossref] [PubMed]
- Bieze M, Busch OR, Tanis PJ, et al. Outcomes of liver resection in hepatocellular adenoma and focal nodular hyperplasia. HPB (Oxford) 2014;16:140-9. [Crossref] [PubMed]
- Yan Y, Cai X, Geller DA. Laparoscopic Liver Resection: A Review of Current Status. J Laparoendosc Adv Surg Tech A 2017;27:481-6. [Crossref] [PubMed]
- Filmann N, Walter D, Schadde E, et al. Mortality after liver surgery in Germany. Br J Surg 2019;106:1523-9. [Crossref] [PubMed]
- Nomi T, Fuks D, Kawaguchi Y, et al. Learning curve for laparoscopic major hepatectomy. Br J Surg 2015;102:796-804. [Crossref] [PubMed]
- Brown KM, Geller DA. What is the Learning Curve for Laparoscopic Major Hepatectomy? J Gastrointest Surg 2016;20:1065-71. [Crossref] [PubMed]
- Chiow AK, Lee SY, Chan CY, et al. Learning curve in laparoscopic liver surgery: a fellow's perspective. Hepatobiliary Surg Nutr 2015;4:411-6. [PubMed]
Cite this article as: Sucher R, Guice H, Recknagel S, Sucher E, Semmling K, Lederer A, Rademacher S, Scheuermann U, Seehofer D. Analysis of open and laparoscopic liver resections in a German high-volume liver tumor center. Laparosc Surg 2021;5:3.


