
Green indocyanine in minimally invasive spleen preserving distal pancreatectomy for insulinoma: report of two cases
Introduction
Nowadays, intraoperative use of fluorescence with indocyanine green (ICG) is widely accepted in many fields of general surgery (1-6) but only few authors (7-9) reported their results about ICG fluorescence in pancreatic neuroendocrine tumors (PNETs). Surgery represents the best option of treatment in the management of pancreatic insulinoma with high rate of surgical cure, but the correct identification of the pancreatic lesion represents the most challenging aspect of minimally invasive technique, whose lack of tactile feedback is still a limit (10-13).
For these reasons near-infrared (NIR) fluorescent imaging was recently introduced, in addition to intra-operative ultrasound (IOUS), as a useful tool for accurate identification of insulinoma. In this study, we describe our first two cases of minimally invasive spleen preserving distal pancreatectomy (SPDP) for insulinoma with ICG administration. Our aim is to demonstrate reliability of NIR technique in insulinoma localization during minimally invasive pancreatic surgery.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/ls-20-128).
Case presentation
From December 2019 to January 2020, two patients underwent spleen preserving distal pancreatectomy (SPDP) with ICG intraoperative detection of the insulinoma. The first was treated with laparoscopic approach, the second with robotic assisted technique.
Intraoperative details, technical surgical aspects and postoperative outcomes are all described; a comparison with literature is also reported.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (as revised in 2013). All patients gave their formal consent before surgical procedure.
Clinical case 1: laparoscopic SPDP
Male patient, aged 63 years; the pathological personal history (PPH) included obesity (Class I) and atrial fibrillation. Laboratory examination confirmed the clinical suspect of insulinoma. Screening test for multiple endocrine neoplasia type 1 (MEN1) was negative.
Before surgery, imaging localization study is performed with magnetic resonance imaging (MRI) (Figure 1) which described the presence of a 13 mm lesion at the posterior side of the pancreatic body closed to the superior mesenteric vein (SMV), in absence of vascular infiltration. Endoscopic ultrasound (EUS) confirmed a uT1cN0 lesion with 2 mm distance from the main pancreatic duct (MPD) and at guided fine-needle aspiration (FNA) cytology, a neuroendocrine tumor with MIB1 <1% resulted.
The patient was positioned in a 20° reverse Trendelenburg position with a lateral 30° right flank tilt. Four trocars were placed: the optical trocar in the midline above the umbilical scar with a 30° camera Olympus Visera Elite II® with dedicated NIR xenon light source (Olympus Italia S.r.l), one 12-mm trocar to the left flank and one 5-mm trocar to the right flank, lateral to the rectus muscles; the last one 5-mm trocar was positioned in the midline below the xiphoid process.
The pneumoperitoneum was established with a Verres-needle. The gastrocolic ligament was then sectioned, and the lesser sac opened in order to completely expose the anterior face of the pancreas. The stomach was retracted with a blunt dissector by a 5 mm epigastric port. Dissection was carefully continued using the mono-polar hook and ultrasonic dissector, following the inferior margin of the pancreas; the spleno-mesenteric confluence was exposed, and a clear plane of dissection from the anterior surface of SMV was obtained.
The retro-pancreatic tunnel was then started: the pancreatic neck was detached from the spleno-mesenteric confluence, and passed with grasping forceps and with a rubber tape, so by lifting the pancreatic neck, the splenic vein was isolated.
Laparoscopic IOUS was performed and all pancreatic parenchyma was examined. The pancreatic lesion of 13 mm at the posterior side of the pancreatic body, visualized at the MRI, was confirmed. The lesion did not compress the MDP but with only 2 mm distance.
A dosage of 25 mg of ICG was administered intravenously and after few seconds the NIR imaging camera was activated. An area of higher fluorescence was detected at the posterior border of pancreatic body corresponding to the neuroendocrine lesion (Figure 2).
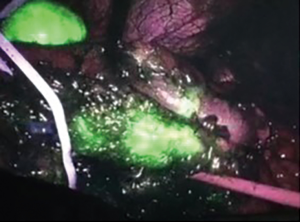
A distal pancreatectomy was then performed with a powered endostapler (SigniaTM MedTronic Italia SpA) (2 linear, medium thick, 60 mm cartridge). A meticulous dissection was carried on by lifting the pancreas stump and separating the posterior side of the gland from the splenic vessels and from retroperitoneal surface. Small branches from the pancreas to splenic vein were dissected with harmonic scalpel or by ligation with hemostatic clip. The pancreatic specimen was then placed into an endobag and removed thoroughly umbilical port. An intraoperative inferior splenic vein branch bleeding was promptly controlled with clip and a subsequent limited splenic inferior pole infarction resulted (Figure 3).
Hemostatic material (Tachosil®) and a Penrose drain was placed at the pancreatic stump. The operative time, skin to skin, was 228 minutes. Blood loss was 200 cc. Oral intake was started at 1st post-operative day (POD). Serum amylases was normal in each sample. Amylases from drainage fluid was performed at POD 1st, 3rd, and 5th, resulting respectively in 1,904, 158, 233 U/L. At 3° POD the patient presented abdominal pain at left flank and a Computed Tomography (CT) scan was performed: a fluid collection near the pancreatic stump (40 mm ×35 mm ×150 mm) was reported. A conservative management was adopted; output from the abdominal drains showed a gradual decline as the amylases level and on 9th POD, drain was dropped off.
The patient was successfully discharged at 10th POD. The histological examination confirmed NET G1 Ki-67 <2%. Six lymph nodes were harvested, all negative for metastases and with clear margins.
One month after surgery, CT scan revealed the presence of two pseudocysts near the pancreatic tail. The patient was asymptomatic and a follow-up program was planned.
Clinical case 2: robotic SPDP
Female patient, 30 years old; PPH included obesity (Class I).Laboratory examination confirmed the clinical suspect of insulinoma. Screening test for MEN1 was negative.
MRI and EUS were performed, and a 14 mm lesion was detected at the pancreatic isthmus. No signs of vessels or MPD invasion, 2 mm distance from MPD; a 6 mm lymph node was described at the tail of the pancreas. The FNA cytology revealed a NET with MIB1 2%.
A robotic SPDP was performed, with the DA VINCI SI surgical system (Intuitive Surgical Inc.®). Robotic trocars placement is reported in Figure 4.
Da Vinci SI platform is lacking of an integrated NIR imaging system, so we decided to overcome this by using the laparoscopic camera Olympus Visera Elite II® with the dedicated NIR xenon light source (Olympus Italia Srl).
After the identification of the pancreatic lesion with IOUS, confirming EUS findings, a total of 25 mg of ICG fractioned into 5 bolus of 5 mg per minute was administered intravenously and after a time of 20 minutes the NIR laparoscopic camera is introduced into the operating field through the assistant port. In this case the fluorescence signal spread throughout the abdomen, thus we failed the exact localization of the pancreatic insulinoma and any local lymph node (Figure 5).
However, IOUS permitted to establish the appropriate surgical margins of resection. The pancreatic transection is performed by powered endostapler (SigniaTM MedTronic Italia SpA) (one linear, medium thick, 60 mm cartridge reinforced with Seamguard®). Accurate dissection of the splenic vessels from the posterior pancreatic margins was performed with monopolar hook. A Penrose drain was placed near the pancreatic stump. The operative time, skin to skin, was 225 min. Blood loss was 100 cc. Oral intake was started at 1° POD. Serum amylases was normal in each sample; the dosage of amylases from drainage fluid at 1st, 3rd and 5th POD was respectively to 8,164, 614, 1,353 U/L.The patient was discharged at 9°POD with the drain. At 18° POD after a complete resolution of drain’s fluid the drain was removed.
The histological examination confirmed NET G2 Ki-67 4% with negative surgical margins.
Any recurrences are recorded in 9 months follow-up.
Discussion
PNETs are a rare entity and insulinoma is the most common functional PNETs (10), less than 10% are malignant (14) and only in 5% of cases they are related to Multiple Endocrine Neoplasia type 1 (MEN1) (11).
In 2019 Andreassen et al. reported that the most effective non-invasive diagnostic methods for primary localization of pancreatic insulinoma are MRI and CT (15). Contrast CT scan has a sensitivity and specificity respectively equal to 82% and 96% (10). It permits to evaluate local extension, vascular and lymph nodes (LN) involvement. EUS has a higher sensitivity to detect small tumors (<20 mm), especially for those located at the head of the pancreas (14,16), and eventual regional lymph node metastasis (10); biopsy in case of uncertain diagnosis can be also performed. In a recent review Walczyk et al. reported that the sensitivity of EUS for detection of insulinoma may be higher than that of CT (84.2% vs. 31.6%) (16).
European guidelines for PNETs management were proposed in 2016 by European Neuroendocrine Tumor Society (ENETS) (17). Surgery was defined as the gold standard of treatment for symptomatic sporadic insulinoma with dimension more than 20 mm, presenting a significant tumor growth and aggressive characteristics (infiltration of MPD, vascular infiltration and compressive symptoms) (18). The size of the tumor and the relation with the MPD and splenic vessels, as well as the occurrence of multiple lesions, are crucial elements in determining the correct surgical procedure.
In particular for superficial small (<20 mm) insulinoma with a minimum distance of 3 mm from MPD, pancreatic spearing enucleation is recommended as treatment of choice (10,19); lymphadenectomy is not generally required, because of high percentage of benignity (14,17). Enucleation in fact, is associated with a significantly reduced incidence of exocrine and endocrine insufficiency, but on the other hand it presents a higher risk of post-operative pancreatic fistula (POPF) (19).
Laparoscopic pancreatic distal resection is well recognized as a safe procedure (13,18); patients with benign tumors located at pancreatic body or tail, with a distance from MPD less than 3 mm, can better benefit from laparoscopic distal pancreatectomy (19).
Exact localization of PNETs represents one of the most challenging aspects of the surgical procedure; among the drawback of the laparoscopic technique, the lack of tattle feedback makes the recognition of the pancreatic lesion more difficult. As a consequence, laparoscopic IOUS plays an important role in PNETs intraoperative localization, with a sensitivity of 90–96.9% (16,20). As Wu et al. confirmed in 2017, laparoscopic IOUS reinforces the feasibility of laparoscopic resection for insulinoma and its strength as a minimally invasive procedure (20).
To improve minimally invasive pancreatic surgery, an important role is also played by real time fluorescence, with the aim of a correct lesion localization.
ICG is a water-soluble fluorophore that binds to the plasma protein after intravenous administration. ICG is safe, nontoxic and doesn’t present radioactive proprieties. The half time life is 150–180 seconds and the metabolism are exclusively hepatic. When excited by NIR light (800 nm) it emits a fluorescent signal. Thanks to this characteristic represents an emerging promising intraoperative technology suitable especially for minimally invasive surgery (2).
In particular in the contest of pancreatic surgery the exact localization of the tumor permits to obtain free resection margins, with high rate of curative treatment in the context of parenchyma spearing resection. The intra operative use of ICG fluorescence is employed to assist the surgeon during laparoscopic procedure in combination with laparoscopic IOUS.
Nowadays, literature about the application of ICG for pancreatic insulinoma is lacking and only few authors reported their preliminary experience (7-9).
The Verona Group (Paiella et al.) (7) in 2017 published the results of COLPAN study. They described 10 cases of patients underwent laparoscopic pancreatic surgery and documented 100% NIR visualization rate for PNETs. They administered sterile ICG powder 25 mg diluted in 10 cl of sterile saline solution with a final stock concentration of 2.5 mg/mL. Then 5 boli of 2 cl were given. The peak of tumor fluorescent visualization can be reached 20 minutes after the last bolus administration of ICG. They noticed also that ICG could concentrate in peripancreatic lymph nodes, to be considered a guidance to perform lymphadenectomy in selected cases presenting high suspicion of metastasis at preoperative imaging. At the end of the study Paiella et al. concluded that a single intravenous bole administration of high dosage of ICG (25 mg) could be the better solution to well delimited the area of higher fluorescence corresponding to functional PNETs.
Constantinescu et al. (9) reported a case report of an insulinoma located at the head of the pancreas treated by standard laparotomic pylorus preserving-pancreaticoduodenectomy. ICG was applied to assess a possible multicentric insulinoma localization or secondary lymph nodes involvement; they administered, as the Verona group, five consecutive boluses of 2.5 mg of ICG and they obtained a delimited area of high fluorescence corresponding to the lesion previously described.
In 2018 Shirata et al. (8) hypothesized that hypervascular lesion can manifest fluorescence because of their higher vascularity than the normal surrounding pancreatic parenchyma. With a dosage of only 2.5 mg intravenous ICG administered to 23 patients during laparoscopic or open pancreatic resection, they obtained significantly higher values of fluorescent intensity in all 5 PNETs.
Standard protocol for application of ICG for intraoperative detection of functional PNETs needs to be defined due to different dosages and modality of administration of ICG reported in literature. According to our two cases, the intravenous 25 mg bolus of ICG administration seems to be the best modality to reach the high peak of fluorescence and to identify the PNETs lesions. The administration of five consecutive boli didn’t allow in our case, to identify the lesion due to a spread of fluorescence in all the surgical field.
None intraoperative adverse effects occurred and the two patients reported biochemically pancreatic leak (grade A fistula), both successfully conservative managed and resolved. Tumor margins were free and at 9 months-follow up no recurrences are recorded.
Conclusions
To the best of our knowledge, real time ICG fluorescence appears to be a safe, feasible, and achievable technique for intraoperative detection of functional PNETs. NIR fluorescence with ICG represents a useful tool, in addition of IOUS, for the intra-operative minimally invasive pancreatic decision making of insulinoma treatment. To reach the best insulinoma fluorescence imaging visualization we propose a single endovenous bolus administration of ICG at the high dosage of 25 mg. A division of ICG 25 mg in multiple boli didn’t identify, in our case, the PNET lesion. Further studies to assess the best dosage of administration in order to reach the best result.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Nadia Russolillo) for the series “Amplifying the voices of women surgeon scientists” published in Laparoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/ls-20-128
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-128). The series “Amplifying the voices of women surgeon scientists” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (as revised in 2013). All patients gave their formal consent before surgical procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Manen L, Handgraaf HJM, Diana M, et al. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol 2018;118:283-300. [Crossref] [PubMed]
- Spinoglio G, Bertani E, Borin S, et al. Green indocyanine fluorescence in robotic abdominal surgery. Updates Surg 2018;70:375-9. [Crossref] [PubMed]
- Daskalaki D, Aguilera F, Patton K, et al. Fluorescence in Robotic Surgery. J Surg Oncol 2015;112:250-6. [Crossref] [PubMed]
- Osayi SN, Wendling MR, Drosdeck JM, et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 2015;29:368-75. [Crossref] [PubMed]
- Blanco-Colino R, Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 2018;22:15-23. [Crossref] [PubMed]
- Baiocchi GL, Diana M, Boni L. Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: State of the art and future directions. World J Gastroenterol 2018;24:2921-30. [Crossref] [PubMed]
- Paiella S, De Pastena M, Landoni L, et al. Is there a role for near-infrared technology in laparoscopic resection of pancreatic neuroendocrine tumors? Results of the COLPAN "colour-and-resect the pancreas" study. Surg Endosc 2017;31:4478-84. [Crossref] [PubMed]
- Shirata C, Kawaguchi Y, Kobayashi K, et al. Usefulness of indocyanine green-fluorescence imaging for real-time visualization of pancreas neuroendocrine tumor and cystic neoplasm. J Surg Oncol 2018;118:1012-20. [Crossref] [PubMed]
- Constantinescu DP, Constantinescu MI, Ciocan RA, et al. Near infrared light examination as part of the management of sporadic pancreatic head insulinoma: Case report. Int J Surg Case Rep 2019;64:35-40. [Crossref] [PubMed]
- Jeune F, Taibi A, Gaujoux S. Update on the Surgical Treatment of Pancreatic Neuroendocrine Tumors. Scand J Surg 2020;109:42-52. [Crossref] [PubMed]
- Antonakis PT, Ashrafian H, Martinez-Isla A. Pancreatic insulinomas: Laparoscopic management. World J Gastrointest Endosc 2015;7:1197-207. [Crossref] [PubMed]
- Zhang J, Jin J, Chen S, et al. Minimally invasive distal pancreatectomy for PNETs: laparoscopic or robotic approach? Oncotarget 2017;8:33872-83. [Crossref] [PubMed]
- Cesaretti M, Bifulco L, Costi R, et al. Pancreatic resection in the era of laparoscopy: State of Art. A systematic review. Int J Surg 2017;44:309-16. [Crossref] [PubMed]
- Doi R. Determinants of surgical resection for pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci 2015;22:610-7. [Crossref] [PubMed]
- Andreassen M, Ilett E, Wiese D, et al. Surgical Management, Preoperative Tumor Localization, and Histopathology of 80 Patients Operated on for Insulinoma. J Clin Endocrinol Metab 2019;104:6129-38. [Crossref] [PubMed]
- Walczyk J, Sowa-Staszczak A. Diagnostic imaging of gastrointestinal neuroendocrine neoplasms with a focus on ultrasound. J Ultrason 2019;19:228-35. [Crossref] [PubMed]
- Falconi M, Eriksson B, Kaltsas G, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016;103:153-71. [Crossref] [PubMed]
- Wong KP, Tsang JS, Lang BH. Role of surgery in pancreatic neuroendocrine tumor. Gland Surg 2018;7:36-41. [Crossref] [PubMed]
- Role of minimally invasive surgery in the treatment of pancreatic neuroendocrine tumors. In: Boggi U (ed). Minimally invasive surgery of the pancreas. Milan: Springer-Verlag Mailand, 2018.
- Wu M, Wang H, Zhang X, et al. Efficacy of laparoscopic ultrasonography in laparoscopic resection of insulinoma. Endosc Ultrasound 2017;6:149-55. [Crossref] [PubMed]
Cite this article as: Francescato A, Mullineris B, Pecchini F, Gozzo D, Piccoli M. Green indocyanine in minimally invasive spleen preserving distal pancreatectomy for insulinoma: report of two cases. Laparosc Surg 2021;5:39.






