
Pure laparoscopic major hepatectomy facilitated with an application of Gelport™: a single-center case series
Introduction
Laparoscopic liver resection (LLR) has been becoming widely accepted (1,2). In Japan, national health insurance has covered the medical fee for anatomical liver resection since 2016, in addition to left lateral sectionectomy and local liver resection, which have been covered since 2010 (3).
LLR can be performed as a pure laparoscopic procedure, hand-assisted laparoscopic surgery (HALS) or hybrid procedure (4,5). Each procedure has its own merits and drawbacks (6-9). Pneumoperitoneum is accepted as a procedure associated with a reduced intraoperative blood loss. However, since parenchymal transection is performed under direct vision during the hybrid procedure, these advantages of pneumoperitoneum are not available with this procedure, in contrast to the pure laparoscopic procedure and HALS.
The advantages associated with HALS are the facilitation of liver mobilization, tactile feedback and ease of controlling emergent bleeding (8). Furthermore, in hepatectomies with large parenchymal resection, the hand port can be used as the extraction site of the resected specimens without extending the incision.
We herein report our procedure of laparoscopic major hepatectomy, which includes the advantage of both pure LLR and HALS.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ls-20-17).
Methods
We have conducted 9 cases of anatomical resection with this technique. First, we report the detail of surgical technique in each type of anatomical resection. Second, we reviewed intra- and post-operative outcomes in the above-mentioned 9 consecutive cases. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the Nagasaki University Hospital (decision number 19102143), and informed consent was obtained from all of the participating patients.
Patients’ characteristics
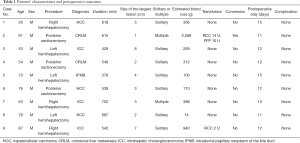
Characteristics of the patients were shown in Table 1. All of the patients were male. Age of the patients was between 35 and 87. Indications of hepatectomy were as follows: hepatocellular carcinoma in 3 patients, intrahepatic cholangiocarcinoma 3 patients, colorectal liver metastasis in 2 patients, and intraductal papillary neoplasm of bile duct in 1 patient. The largest size of the lesion in the patients was 8 cm. Two patients had two lesions in the same lobe.
Full table
Right hemihepatectomy
Patients are placed in the supine position with split legs. First, an 8-cm midline incision is made over the umbilicus; and a Gelport (Applied Medical, Rancho Santa Margarita, CA, United States) is placed (Figure 1A). A 12-mm trocar is inserted through the Gelport for insufflation and an initial inspection. Subsequently, 2 trocars (5 and 12 mm) are placed in the right and left upper quadrant, respectively. Furthermore, a 5-mm trocar is placed in the epigastric area (Figure 1B). To reduce the possibility of tumor cells spreading, the right branch of the portal vein is transected before mobilization of the liver in cases with HCC. Before starting hilar dissection, the hepatoduodenal ligament is encircled for the Pringle maneuver by a hand-assisted procedure (Figure 1C). After establishing the Pringle maneuver, the hand is removed. Following cholecystectomy, the right hepatic artery and the right branch of the portal vein are isolated by a pure laparoscopic procedure (Figure 1D). The demarcation line is ensured by clamping the right hepatic artery and the right branch of portal vein. In addition, intraoperative ultrasonography is performed to clarify the flow into the left liver, the location of the tumor and the relationship between the tumor and vessels. We use the same ultrasound probe as that used in an open procedure; the probe is inserted through the hand-port. Due to the direct manipulation of the probe by the surgeon, meticulous investigation is more easily accomplished than with an ultrasound probe for laparoscopic usage. Subsequently, the right hepatic artery is transected between clips (Hem-o-lok®; Morrisville, NC, USA). The right branch of the portal vein is transected with a vascular stapler (Powered ECHELON FLEX® 7; Ethicon, New Alexandria, PA, USA).

After the transection of the artery and portal vein, mobilization of the liver is performed by HALS. After sufficient mobilization of the liver, the inferior vena cava is exposed. Short hepatic veins are transected between clips; the small vessels are managed with a vessel sealing system (LigaSure; Covidien, Minneapolis, MN, USA). After sufficient exposure of the caudal side of the inferior vena cava, the hepatocaval ligament (Makuuchi’s ligament) is transected using a vascular stapler. Subsequently, the connective tissue around the right hepatic vein is dissected, and the vein is encircled. Parenchymal resection was performed using a Cavitron Ultrasonic Surgical Aspirator (CUSA) a sealing device (LigaSure) and a saline-linked ball-shaped dissecting sealer. During parenchymal resection, pneumoperitoneum was set at 12 mmHg. With sufficient exposure, the vessels are transected with a clipping or sealing device with a small width. After creating sufficient space by dissecting the surrounding parenchyma, the right hepatic duct is encircled with absorbable ligature and then transected using a vascular stapler (Powered Echelon FLEX®, white cartridge; Ethicon, New Alexandria, PA, USA).
To resect the dorsal and deep areas of the parenchyma, a hanging maneuver is effective. The Pringle maneuver is used with clamping periods of 15 minutes separated by at least 5-minute periods of declamping. The total clamping duration is 65 minutes. After completing parenchymal resection, the right hepatic vein is transected using a vascular stapler. To retrieve the resected specimen, a retrieval bag is inserted. The bag, including the specimen, is extracted through the incision cross the navel without any extension.
Figure 2 shows an appearance of postoperative scar a 6-month after surgery.
Left hemihepatectomy
In left hemihepatectomy, mobilization of the right lobe is not necessarily performed. As a result, role of HALS is relatively limited compared with right hemihepatectomy or posterior sectionectomy. Whereas, HALS is considered effective in case with soft tumor, huge tumor or invasive tumor on the surface of the liver, because mobilization of the left lobe only with forceps in such cases is not always smooth and safe. In such cases, manipulation of the liver with HALS is useful in left hemihepatectomy as well as right hemihepatectomy and posterior sectionectomy. For vascular management, extrahepatic Glissonean approach could be an option in case with peripheral tumors those are not located adjacent to umbilical portion.
Posterior sectionectomy
Basic process of the procedure is similar to right hemihepatectomy. For vascular management, extrahepatic Glissonean approach is the first option. Following cholecystectomy, we isolate the right posterior Glissonean pedicle. With clamping posterior Glissonean pedicle, demarcation line between posterior and anterior section is recognized and marked. Also, inflow to the anterior sector should be checked with ultrasonography. When there is sufficient space for inserting vascular stapler, we transect the posterior branch by vascular stapler. Whereas, when it is difficult to transect the posterior Glissonean pedicle before parenchymal transection, it is also possible to transect the parenchyma with clamping posterior Glissonean pedicle as an inflow occlusion.
Statistical analysis
This study is a case series which contains fundamental statistics only. The data are indicated as the median values and range.
Results
The type of hepatectomy conducted with this technique was as follows: right hemihepatectomy in 3 patients, left hemihepatectomy in 3 patients, and posterior sectionectomy in 3 patients. Perioperative outcomes were shown in table 1. Median operation duration 548 minutes (range, 378–702 minutes). Although statistical analysis was not conducted because of small number of cases, operation durations of left hemihepatectomy tended to be short compared with other two types of procedure. The median volume of estimated blood loss was 312 g (range, 14–5,088 g). The patient with estimated blood loss of 5,088 g underwent conversion from LLR to conventional open procedure. The reason of conversion in the case was unideal exposure of the operative field during the parenchymal transection. Two patients underwent transfusion. Besides one patient with conversion to open procedure, another patient who was 87 years old with intrahepatic cholangiocarcinoma underwent transfusion as a result of blood loss of 940 g. None of the patients developed postoperative complications. Postoperative hospital stay was between 10 and 15 days.
Discussion
We herein report our procedure used in laparoscopic major hepatectomy. This procedure includes the merits of both a pure laparoscopic procedure and HALS. Most of the procedure was performed via pure laparoscopic methods (about 90%), and the remaining 10% of the procedure was performed via HALS.
In contrast to lateral sectionectomy and local liver resection, a bigger incision is necessary for the extraction of the resected specimen in major hepatectomy. The size of the hand port is considered sufficient to extract even the hemiliver in most cases. Although the incision to extract the specimens is typically made after the completion of hepatectomy, the incision for extraction was also used as the hand port and was made at the start of the procedure. By establishing the hand port at the beginning of surgery, various advantage of HALS can be achieved at any stage of surgery when necessary. Cosmesis is not a primary objective of this procedure; however, the small postoperative scar cross the navel is considered patient-friendly.
Although devices for intraoperative ultrasonography in laparoscopic procedures have been established, the handling of these devices for meticulous intraabdominal examinations is not always as easy as the handling of an ultrasound probe during open surgery. In our technique, which includes a hand-assisted procedure, intraoperative ultrasound can be performed using a probe introduced through the hand port with the same quality and dexterity as open procedures. Although such maneuverability might be considered a minor issue, intraoperative ultrasonography through the hand port is quite useful, representing another merit of HALS. As another merit, we have experienced the usefulness of hand-assist during adhesiolysis in the case with episodes of previous upper abdominal surgery including hepatectomy. We consider that our procedure is also effective in major hepatectomy in patients with previous abdominal surgery.
According to the recommendation for LLR in a report from the Second International Consensus Conference, HALS can be used to manage intraoperative difficulties that are encountered, and it can decrease the frequency of conversion to a full open incision (10). Furthermore, HALS is considered to be beneficial for large lesions, posterior lesions, donor hepatectomy. In this case series, 3 patients had lesions larger than 5 cm, which were located on the surface of the liver. During the manipulation of the liver, incidental rupture or destruction of tumor should be avoided. HALS is beneficial for mobilizing the liver with tumors on the surface thanks to delicate manipulation by hands compared to laparoscopic devices.
With recognizing the merit of HALS such a delicate manipulation of the liver, we have reported efficacy of hybrid procedure including mobilization of the liver by HALS in donor hepatectomy (11). In donor hepatectomy, the median duration of the mobilization of the liver by HALS was 26 minutes. Also, we have reported that our hybrid procedure can be conducted in any type of hepatectomy (6). Whereas, hybrid procedure does not offer an advantage of pneumoperitoneum with respect to diminishing intraoperative blood loss. The procedure in this report therefore offers both the advantages of pure laparoscopic procedure and HALS. In addition to the recommendation as mentioned-above, HALS is recommended for the training of surgeons in major LLR. Indeed, HALS can be used as a bridge from open procedure or hybrid procedure and pure laparoscopic procedure. However, at the same time, surgeons do not have to abandon the merits of HALS especially in hepatectomy for patients with large lesions or posterior lesions. Depending on the patients’ characteristics as well as surgeons’ or institutes’ experiences, advantages of previously proposed techniques should be utilized and maximized. It will widen the application of laparoscopic anatomical resection in a safety manner.
If the merits of HALS can be achieved without compromising the advantages of 100% pure laparoscopic hepatectomy, this combined procedure may offer additional advantages over a pure laparoscopic procedure. Given the present findings, we consider this procedure not just a bridge to 100% pure laparoscopic hepatectomy but a reasonable approach utilizing the merits of both the pure laparoscopic procedure and HALS especially for complicated major hepatectomy.
Acknowledgements
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ls-20-17
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ls-20-17
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-17). SE serves as the unpaid editorial board member of Laparoscopic Surgery from Sep 2020 to Aug 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the Nagasaki University Hospital (decision number 19102143), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wakabayashi G, Cherqui D, Geller DA, et al. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2014;21:723-31. [Crossref] [PubMed]
- Wakabayashi G. Systematic reviews from the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2015;22:325-6. [Crossref] [PubMed]
- Takahashi Y, Katagiri S, Ariizumi SI, et al. Laparoscopic Hepatectomy: Current State in Japan Based on the 4th Nationwide Questionnaire. Gastroenterol Res Pract 2017;2017:6868745 [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Koffron AJ, Kung RD, Auffenberg GB, et al. Laparoscopic liver surgery for everyone: the hybrid method. Surgery 2007;142:463-8; discussion 468.e1-2. Erratum in: Surgery 2008;143:301. [Crossref] [PubMed]
- Soyama A, Takatsuki M, Adachi T, et al. A hybrid method of laparoscopic-assisted open liver resection through a short upper midline laparotomy can be applied for all types of hepatectomies. Surg Endosc 2014;28:203-11. [Crossref] [PubMed]
- Hasegawa Y, Koffron AJ, Buell JF, et al. Approaches to laparoscopic liver resection: a meta-analysis of the role of hand-assisted laparoscopic surgery and the hybrid technique. J Hepatobiliary Pancreat Sci 2015;22:335-41. [Crossref] [PubMed]
- Cardinal JS, Reddy SK, Tsung A, et al. Laparoscopic major hepatectomy: pure laparoscopic approach versus hand-assisted technique. J Hepatobiliary Pancreat Sci 2013;20:114-9. [Crossref] [PubMed]
- Cheek SM, Sucandy I, Geller DA. Hand-assisted laparoscopic left hepatectomy: how I do it. J Hepatobiliary Pancreat Sci 2016;23:E30-E32. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Eguchi S, Soyama A, Hara T, et al. Standardized hybrid living donor hemihepatectomy in adult-to-adult living donor liver transplantation. Liver Transpl 2018;24:363-8. [Crossref] [PubMed]
Cite this article as: Soyama A, Natsuda K, Hidaka M, Adachi T, Ono S, Hamada T, Kugiyama T, Hara T, Eguchi S. Pure laparoscopic major hepatectomy facilitated with an application of Gelport™: a single-center case series. Laparosc Surg 2021;5:29.



