
Laparoscopic transperitoneal adrenalectomy—indications and technical aspects
Introduction
Since its first description in 1992, trans-peritoneal laparoscopic adrenalectomy has become the gold standard for managing most adrenal pathology (1). Prior to this, the only option for patients requiring adrenalectomy was painful and often morbid open surgery either via anterior laparotomy or the posterior approach through the loin. Compared with open surgery, laparoscopy affords a better view of the peri-adrenal region and the relatively compact adrenal gland. This, combined with the familiarity of the transperitoneal approach, has led to its widespread adoption. Furthermore, decreased blood loss, shorter hospital stay due to decreased post-operative pain, and reduced likelihood of complications (2-4), have meant that the transperitoneal laparoscopic approach is the most commonly employed technique for adrenalectomy. Subsequently, retroperitoneoscopic adrenalectomy (a modification of the posterior approach) popularised by Walz (5,6) and robotic-assisted surgery (7-9) have also gained popularity. Thus, a patient requiring adrenal surgery currently may in theory have their procedure tailored to the type and extent of adrenal pathology found at presentation. According to voluntary surgical registries such as the United Kingdom Registry of Endocrine and Thyroid Surgery (UKRETS) and Eurocrine®, in general, adrenal surgery is very safe with an overall mortality of <0.5% (10,11). Therefore the focus should be on minimising morbidity (which is less well quantified) and maximising quality of patient care for what is quite a rare operation. This article will focus on the preoperative considerations, indications and technical aspects of trans-peritoneal laparoscopic adrenalectomy.
Preoperative considerations
When managing any patient with an adrenal tumour, it is necessary to determine the following:
- Is the tumour functioning or non-functioning and is the diagnosis correct?
- If the tumour is functioning, has the patient’s physiology been rendered safe for surgery to proceed?
- Is the patient’s radiology contemporaneous?
- If surgery is to take place are the correct personnel and equipment available?
- Is surgery definitely indicated?
Assuming that the above factors have all been addressed, the surgeon must then decide whether the pathology is best dealt with via open surgery or a minimally invasive approach. Patient factors, tumour factors, and surgeon factors will dictate the best surgical strategy in a given situation.
Patient factors
Although they may not preclude minimally invasive surgery, the following factors may make it more technically challenging: morbid obesity [body mass index (BMI) >40] may mean that the patient is not a candidate for retroperitoneal surgery because they cannot lie prone, therefore a transperitoneal approach may be most suitable. Hepatic steatosis will make transperitoneal right adrenalectomy much more difficult. Adhesions from previous extensive abdominal surgery in the upper abdomen may steer the surgeon towards a retroperitoneoscopic approach, particularly if the tumour is small (<5 cm) and/or bilateral.
Tumour factors
The critical question with respect to the tumour is the likelihood of malignancy. Size, appearance and density in Hounsfield units (HU) on non-contrast pre-operative adrenal CT scan will usually provide most of the necessary information. Thus homogenous, low-density tumours (<10 HU), particularly if <4 cm in maximal diameter are best managed with endoscopic excision if functioning (12). Indeterminate tumours between 4 and 6 cm with no evidence of invasion may be approached in similar fashion (12,13). Above 6 cm, the rate of malignancy is 1 in 4 and so the decision to undertake minimally-invasive surgery is more nuanced (13-15). It will depend upon surgeon experience, the absence of any suspicious features on imaging and a clear plan to convert to open surgery if it becomes clear intra-operatively that the tumour is malignant. The presence on imaging of local invasion into adjacent structures [liver, kidney and inferior vena cava (IVC) on the right; kidney, pancreas and spleen on the left], lymph node or distant metastatic disease, and tumour thrombus are suggestive of adrenocortical cancer (ACC). Minimally-invasive surgery is contraindicated in this scenario because only open adrenalectomy with en bloc excision of other involved structures will afford the patient any chance of cure (15). Invasion of the renal vessels by tumour is more frequent than direct involvement of the renal parenchyma and may be observed in ACC, metastasis and malignant paraganglioma. If present, it will also mandate en bloc excision of kidney with the tumour usually via an open approach.
Surgeon factors
The rarity and potential complexity of adrenal disease means that it can be challenging for low volume centres to provide a comprehensive service to patients requiring adrenalectomy. In the UK, the majority of adrenal surgery is undertaken by surgeons performing a median of one procedure per year (16,17) and the median number performed by contributors to the UKRETS is 6 per annum (10). However, when attempts are made to quantify the learning curve of TLA, it has been demonstrated that competence is attained after approximately 30 procedures (18,19). Therefore, opportunities to train in adrenal surgery will be limited to centres with an adequate caseload of open and minimally-invasive surgery (20). Furthermore, it is becoming increasingly apparent that surgical volume and access to support from other multidisciplinary specialists (e.g., endocrinology, radiology, interventional radiology and pathology) are key determinants of outcomes such as length of stay, complications and readmissions (16,21). The administrative dataset for the recently published ‘Getting it right first time’ (GIRFT) endocrinology report studied outcomes from more than 4,000 adrenalectomies. The study demonstrated that only a third of surgeons (who operated on more than 50% of patients) performed more than 6 procedures per annum. However, readmission rates for open surgery fell from 15.2% to 6.4% between the lowest and highest volume surgeons. There was a similar, non-significant fall for minimally invasive surgery (22). These findings formed the basis of recommendations from the GIRFT report, that adrenal surgeons should perform at least 6 cases each per year and that centres undertaking surgery for adrenal cancer and phaeochromocytoma should perform at least 20 adrenalectomies per annum (23).
Indications and contraindications
Transperitoneal laparoscopic adrenalectomy (TLA) is appropriate for all benign functional adrenal tumours up to nine or ten cm in diameter and for indeterminate and non-functioning tumours >4 cm where there is no evidence of local invasion. It is also indicated for patients with oligometastatic disease involving the adrenal gland provided there is no local invasion to preclude a minimally-invasive approach. Lastly, it is feasible to remove bilateral tumours via the transperitoneal approach although it requires repositioning during the procedure.
Open surgery should be considered the standard approach when ACC is strongly suspected or confirmed, particularly in tumours >6 cm (see above). Between 4 and 6 cm, if there is no local invasion or metastatic lymph nodes, and surgical expertise available, there may be a case for laparoscopic excision (15,24).
Relative contraindications to laparoscopic surgery include the presence of a very large benign tumour (>10 cm) since it can be very challenging to excise such lesions safely and they require a laparotomy to remove them from the peritoneal cavity in any event. The likely presence of extensive adhesions and bilateral tumours, particularly if small, does not mandate the retroperitoneoscopic approach, but it may be a less challenging procedure than a transperitoneal approach.
Technical aspects
Pre-operative preparation
Once the decision to undertake TLA has been confirmed, close liaison with medical endocrinology colleagues is advised in the management of functional tumours (autonomous cortisol-secreting tumours, phaeochromocytoma, primary hyperaldosteronism) to ensure that the patient is medically fit for surgery (peri-operative steroid cover, pre-operative alpha-adrenergic blockade, correction of pre-operative hypokalaemia, respectively). No special dietary or bowel preparation is usually required, but a bariatric diet for 2–3 weeks before surgery is highly effective in reducing liver steatosis in the morbidly obese (25) and it has been shown to reduce technical difficulty and operative time in gallbladder surgery (26). Therefore, it should be considered in those undergoing right-sided surgery, and may be of benefit in all very obese patients. Antibiotic prophylaxis is not routinely used apart from in patients with Cushing’s syndrome due to their relatively immunocompromised state (27). All patients should be risk-assessed for their requirement for mechanical and chemical thromboprophylaxis before surgery and it should be prescribed as per local guidelines. It should be noted that patients with Cushing’s syndrome are also at a greater risk of thromboembolic disease and so careful attention should be paid to chemical prophylaxis in the peri-operative period in this sub-group of patients (28).
Equipment
Standard laparoscopic equipment is required and, according to personal preference, a 0- or 30-degree laparoscope may be employed. The majority of procedures can be completed with 5 mm traumatic forceps (Johan fenestrated grasping forceps or similar) and the desired 5 mm energy device. For the right side, a liver retractor is required, preferably 5 mm.
Patient positioning, equipment and trocars
TLA requires general anaesthesia with full muscle-relaxation. The patient is placed in near full lateral position with the loin positioned over the table-break. Use of a surgical beanbag mattress improves stability and it is imperative that the patient is secured to the table with strapping over the upper torso and pelvis (Figure 1). The dependent leg is flexed slightly and the contralateral leg rests on a pillow placed between the legs. The scrub nurse and laparoscopic stack are situated to the rear of the patient; the assistant and the surgeon stand anterior to the patient.
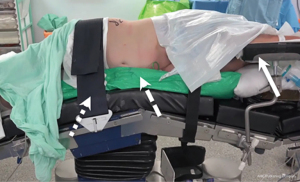
Principals of TLA
Expert working knowledge of the anatomy of the adrenal region is essential. Most notably, the approaches for the right and left sides are distinct. However, the following general principals apply: thorough haemostasis is essential at all times since any bleeding will impair the view by absorbing light from the laparoscope and direct grasping of the adrenal tissue/tumour must be avoided; not only may this cause troublesome bleeding, but also it risks capsular rupture and tumour seeding. The method of entry into the peritoneal cavity will vary according to surgeon preference. Open Hasson entry is preferred by many but will require closure at the end and use of a balloon port or similar to obtain an adequate seal. Alternatively, a Verres needle inserted at Palmer’s point permits CO2 insufflation to 14–15 mmHg followed by entry using a 10+ mm optical plastic bladed trocar, as is the author’s practice. This provides an excellent seal and does not require closure of the deep layers at the end.
Right TLA
The patient is positioned right side up, with the table broken and four ports (two 5 mm and two 10+ mm ports) are used (Figure 2, upper figure). The procedure is best broken-down into the following steps (Figure 3A-3D):

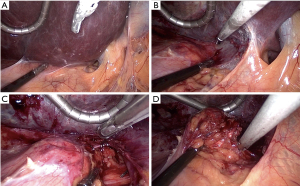
- Retract and mobilise right liver—the liver is retracted cranially and the peritoneal fold between the liver and the tumour (the tumour is almost always visible just deep to the peritoneum), is divided from the lateral border of the IVC to the right triangular ligament of the liver with the preferred energy device.
- Dissect medial border of tumour—The peritoneum is then opened caudally along the lateral border of the IVC to the level of the superior renal pole. This allows the tumour to be retracted laterally to develop the space between it and the IVC. In the process, the short right adrenal vein is exposed, draining from the superior-medial aspect of the gland. At this point, the posterior abdominal wall muscles (quadratus lumborum) should be seen.
- Ligate and divide the adrenal vein—the vein is usually of small caliber. It is clipped with medium-large endoscopic ligaclips and divided with endoscopic shears, taking care to leave a short cuff of vein distal to the clips. This is best facilitated by exposing as much length of the vein as possible.
- Excise the gland—once the vein is divided and the inferior and lateral attachments of the gland, including numerous tiny adrenal arterioles, are divided to fully liberate the gland.
- Place in tissue retrieval bag—the type and size of retrieval bag will depend upon surgeon preference. In general, it will be withdrawn through the lateral-most port and use of robust device is advocated to minimize the risk of the bag rupturing and spilling tumour into the peritoneum. The size of the port incision will need to be extended by an appropriate length to permit this.
- Close ports—the lateral-most port is closed in two/three layers with a size 1 absorbable suture. The other ports (10+ mm a 5 mm) have usually retracted onto the costal margin and rarely need formal closure since the risk of hernia is extremely low. The skin is closed with absorbable subcuticular sutures.
Left TLA
The patient is positioned left side up, with table break, this time three ports are used (one 5 mm port and two 10+ mm ports; see Figure 2, lower figure). The operation proceeds according to the following steps (Figure 4A-4E):
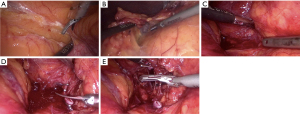
- Mobilise left colon and spleen/tail of pancreas—the aim is to perform a limited medial visceral rotation of the splenic flexure of the colon, spleen and pancreatic tail en masse. This is facilitated by dividing the lateral peritoneal attachments of the colon along the white line of Toldt from the mid-descending colon, cephalad. Dissection is continued to the anterior layer of the lienorenal ligament and proceeds a distance of 1–2 cm from the spleen, until the gastric fundus is just visible, a short distance lateral to cardia of the stomach.
- Open Gerota’s fascia—further dissection and gravity assists the surgeon by allowing the spleen and pancreatic tail to ‘fall’ medially to expose the kidney and perinephric fat, covered in Gerota’s fascia. The tumour is usually visible at this point as a swelling, superior-medial to the upper pole of the kidney. The fascia is opened to allow identification of the adrenal vein as it drains into the left renal vein.
- Locate adrenal vein and ligate/divide—to enable visualisation of the vein, the fat medial to the tumour is carefully teased downward with an endoscopic suction device or endoscopic pledget, whilst very gently retracting the tumour superior-laterally. The vein is usually visible as it drains into the left renal vein. The inferior phrenic vein may be seen draining from superiorly, forming a characteristic ‘Y’ junction with the adrenal vein. Again, the vein is usually quite small and can be clipped with medium-large endoscopic ligaclips and then divided with endoscopic shears. The inferior phrenic vein should also be clipped and divided if it is seen.
- Excise the gland—after the vein has been divided, the tumour is lifted away from the renal hilum with the surgeon’s left grasper and excised by dividing its attachments and blood supply along its superior, inferior and lateral borders. This should expose the superior pole of the kidney and the quadratus lumborum muscle. If the inferior phrenic venous tributary is encountered again, it is dealt with by clipping.
- Place in tissue retrieval bag—the tumour is placed in an endoscopic tissue retrieval bag and removed via the lateral-most port.
- Close ports—the ports are closed as for the right side.
Post-operative care
Oral intake can commence the afternoon/evening of surgery. Pain relief is by either oral analgesics or rarely patient-controlled analgesia infusion. According to UKRETS, the median length of stay following surgery is 3 days (2–5 days) and is largely dictated by the underlying pathology (10). Thus, patients with Cushing’s syndrome will need to be established on oral steroid replacement therapy which necessarily increases this time. Duration of follow-up will depend upon whether patients need to be weaned off steroid replacement therapy or require surveillance for recurrence of pheochromocytoma/paraganglioma. Technical tips and pitfalls to avoid are summarised in Table 1.
Table 1
| Variables | Tips | Pitfalls |
|---|---|---|
| Port placement | For right-sided tumours, place the camera port slightly lower to avoid fatty liver in obese patients | Ensure the lateral port is below the 12th rib and not in the 11 intercostal space |
| Surgical exposure | For left-sided tumours fully mobilise colon/spleen and tail of pancreas, ensuring the fundus of the stomach is visualised, before looking for the left adrenal vein | Avoid grasping the gland/tumour during dissection—bleeding will obscure the view of subsequent dissection |
| Tumour excision | For very vascular tumours, endoscopic clips may be more effective than energy devices for small feeding vessels. Tonsillar swabs are useful to manage troublesome oozing | Excising the periadrenal fat with the adrenal gland, minimises the risk of capsular breech, particularly in Cushing’s disease where tumour rupture risks recurrence in the adrenal bed |
| Peri-operative care | Mitigate the excess risk of infection and thromboembolic disease in patients with Cushing’s syndrome by routine use of antibiotic and thrombo-prophylaxis | Avoid operating on patients until a full endocrinological work-up and MDT discussion have taken place. Small benign non-functioning tumours may not require surgery and patients with a missed functional tumour are at risk of significant peri-operative harm |
MDT, multi-disciplinary team.
Complications
Data from UKRETS and Eurocrine® detail a complication rate of just less than 10% for adrenal surgery (10,11). Conversion to open surgery is observed in 5–10% of cases, with malignant adrenal pathology cited as a significant cause (OR 3.48, P<0.01) (10). Factors such as previous surgery, obesity, presence of phaeochromocytoma and surgeon-volume have also been named as risk factors for conversion. Although laparoscopic adrenalectomy may be associated with complications such as visceral injury, bleeding, injury to the renal vascular pedicle, gas embolism and acute port-site hernia, in reality, most of these are rare, particularly in experienced hands. In addition, right adrenalectomy may be complicated by liver laceration and IVC injury. Whilst the former can usually be managed conservatively with haemostatic agents if required, the latter may necessitate conversion to open surgery if the bleeding cannot be controlled with endoscopic clips. Indeed, unless the operator is experienced at laparoscopic suturing, a swab should be placed over the bleeding area and held with a grasper, whilst a subcostal incision is rapidly undertaken to allow access for suture repair. On the left side, injury to the splenic pedicle, the spleen, gastric fundus and the colon are possible, but can usually be avoided by careful dissection of their respective peritoneal attachments employing a margin of at least 10–15 mm. In the unfortunate event of injury to the left renal vein, a similar approach to that applied for IVC bleeding is advised.
Special situations
Bilateral disease
Bilateral adrenalectomy is most commonly warranted in patients with severe uncontrolled Cushing’s disease, i.e., following failed pituitary surgery; in patients with ectopic adrenocorticotrophic hormone (ACTH) secretion where the source cannot be localised and ablated; and in patients with bilateral phaeochromocytoma, usually on a background of genetic disease, e.g., multiple endocrine neoplasia, von Hippel-Lindau (VHL) syndrome and neurofibromatosis type 1 (NF1). Patients with bilateral adrenal adenomas most often present with asymmetric disease and if they require surgery, they do not usually require simultaneous bilateral adrenalectomy. As stated above, the retroperitoneoscopic approach is an elegant solution if the patient has small bilateral tumours, as is the scenario of Cushing’s disease of whatever cause. If the patient is too obese to lie prone, bilateral TLA will be the next best option. The author’s preference is to perform the right side first, close all ports bar the 5 mm epigastric port, which is left in situ, and covered with a sterile Opsite© occlusive dressing. The patient is then turned onto their right side, taking care not to disturb the port, the Opsite removed, the skin prepped, redraped and the epigastric port used to reinsufflate the abdomen. The three ports required for left TLA are then inserted (first using an optical port for the camera) and the operation proceeds as above. The key message with surgery for Cushing’s disease is that all adrenal tissue must be excised without capsular breach. If any remnant is left in situ, high levels of circulating ACTH will lead to hypertrophy of any remaining adrenocortical tissue and recurrent Cushing’s will ensue. In the case of bilateral adrenalectomy for phaeochromocytoma, patients are usually quite young and so cortical-sparing surgery on one side if possible (see below) should be considered. Bilateral retroperitoneoscopic surgery may be the best option unless the tumour or tumours is/are more than 5 cm in diameter or in the case of a left sided tumour, it is very low and medial to the kidney, making the posterior approach more difficult. In both of these situations, TLA may be the preferred option.
Subtotal adrenalectomy
For patients undergoing bilateral surgery or in those who have had previous unilateral adrenalectomy, cortical-sparing surgery may be appropriate, if feasible (29). In general, this will usually apply to patients with inherited phaeochromocytoma, and may be possible if one of the tumours is no more than 4 cm in diameter (30). The operation commences with total adrenalectomy on the side of the larger tumour. Provided the contralateral tumour is <4 cm (preferably smaller) and located in the medial or lateral portion of the gland, it may be excised and the gland transected with an energy device. Interestingly, post-operative function is still satisfactory if the medial part of the gland and the vein have been removed, due to the rich blood supply of the adrenal. Function is tested in the post-operative period by measurement of a 9 am serum cortisol and/or short synACTHen test. Endocrinology expertise is mandatory in managing such patients following surgery. Long-term steroid independence is described in 57–100% of patients provided at least a third of one gland is left in situ. Recurrent phaeochromocytoma in the remnant is seen in up to 21% of patients and despite background medullary hyperplasia observed in multiple endocrine neoplasia type 2 (MEN2) patients, there does not seem to be an excess risk in these patients compared with those with VHL or NF1 (31).
Metastasectomy
The adrenal gland is a common site for metastasis from solid organ cancers in the abdomen and thorax. They may be diagnosed at presentation (synchronous) or after more than 6-month follow-up (metachronous), either on cross-sectional staging or PET scan. As is the case with adrenal incidentaloma, biochemical screening should be undertaken to exclude phaeochromocytoma and CT thorax/abdomen/pelvis and PET scan should follow to discern if there is metastatic disease elsewhere. The appropriateness of excision will depend upon the performance status of the patient, biology of the primary tumour, prognosis in the setting of distant disease and the options for systemic oncological therapy (32). Classically, resection is favoured in isolated adrenal metastasis, but there is some agreement that oligometastatic disease in more than one site can be treated, provided all lesions are amenable to resection. If adrenalectomy is to proceed, it must be a low morbidity option that can offer significant chances of disease-free survival; therefore a minimally-invasive approach is the most appropriate method (33). Metastases are best dealt with at an early stage (2–3 cm) when an R0 resection is most likely (34), indeed tumour-size <4.5 cm has been shown to be an independent predictor of survival (35). If there is evidence of direct invasion into adjacent organs, as may occur with larger lesions (>4 cm), there should be detailed dialogue between the surgeon, the referring cancer multi-disciplinary team (MDT) and the patient regarding benefits of more extensive open surgery and the appropriateness of proceeding. Due to the rapid progression of certain metastases (e.g., melanoma), it is even more important to have up to date imaging prior to surgery to avoid encountering unresectable disease at operation. It has been shown that prognosis is associated with the primary tumour with renal cell, lung, and colorectal cancer patients exhibiting a median survival of 84, 26 and 29 months respectively (34).
Avoiding pitfalls: achieving an optimal outcome
In most cases, a suboptimal outcome for patient and surgeon can be avoided by careful adherence to the key principals above and avoiding the following.
Incorrect diagnosis
Failure to diagnose a functioning tumour may result in the omission of peri-operative alpha blockade or steroid replacement, leading to serious peri-operative complications. This is best avoided by multi-disciplinary working with endocrinology colleagues and discussion of all operated cases within an Adrenal MDT meeting.
Failure to review radiology
It is imperative that contemporaneous radiology is available and reviewed immediately prior to surgery and the consenting process. Current guidelines for adrenal cancer recommend that all imaging be undertaken within 6 weeks of surgery (15). Imaging that is several months old may result in disease-progression being overlooked in malignant disease. Failure to review radiology before surgery may result in wrong side surgery, particularly if there are errors in imaging reports and correspondence. MDT working and review of imaging and reports reduces the risk of this.
Incorrect equipment
For the most part, the equipment required for standard TLA is widely available but the absence of certain items (e.g., liver retractor for right adrenalectomy, appropriate tissue retrieval bags for larger tumours, vascular endoGIA stapler for ligation and division of the adrenal vein with large tumours) can make surgery more challenging than it needs to be. This is less likely in centres that regularly undertake adrenal surgery.
Wrong personnel
One of the commonly cited reasons for improved outcomes in high volume surgical centres is the value added by the presence of an experienced wider MDT and the contribution made by many parts of the whole team (17,22). Adrenal pathology may occur as part of rare inherited diseases and so concentration of expertise into centres that are familiar with dealing with such conditions is highly likely to improve outcomes. Surgeons undertaking adrenal surgery should also know their outcomes and complications. Membership of specialist associations such as BAETS or European Society and of Endocrine Surgeons and submission of data to registries such as the UKRETS or the European Registry, Eurocrine®, provide a transparent and General Data Protection Regulation (GDPR)-compliant method of data collection and outcome measurement. Conversely, failure to utilise endocrinology expertise in the peri-operative period, or lack of access to anaesthetic teams familiar with the care of rare tumours such as phaeochromocytoma, can impact negatively on patient outcomes and result in unnecessary morbidity and extended post-operative stay in intensive care. Furthermore, failure to audit one’s outcomes leaves no mechanism to monitor quality and safety of patient care.
To conclude, adrenalectomy is a relatively uncommon operation with a clear volume-outcome relationship, especially in the setting of very rare pathology such as phaeochromocytoma and adrenal cancer. As with other complex surgical procedures, there is a clear case for centralisation of adrenal surgery into regional hubs with access to an experienced wider multi-disciplinary team. Continued focus on this will lead to better patient outcomes in the future. It remains to be demonstrated, but advances in technology, such as robotic surgery, may have a role to play in expanding indications and improving surgical outcomes for minimally invasive adrenalectomy.
Summary
- Minimally-invasive adrenalectomy is the surgical approach of choice for most adrenal disease;
- Transperitoneal laparoscopic, retroperitoneoscopic and robotic surgery may lend themselves to particular situations (depending on patient, imaging and tumour factors);
- TLA is contraindicated if there is a strong suspicion of adrenocortical carcinoma especially if there is local invasion of adjacent organs;
- Regardless of specialty, surgeons undertaking TLA should have been adequately trained and should know their caseload and outcomes;
- Adrenal disease is best managed in a multidisciplinary setting in centres with appropriate caseload. There should be close liaison with specialist endocrinologists and radiologists;
- Wider adoption of robotic surgery may widen indications for minimally-invasive adrenalectomy and further reduce morbidity.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Robert Sutcliffe) for the series “Laparoscopic Adrenalectomy” published in Laparoscopic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ls.amegroups.com/article/view/10.21037/ls-21-28/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://ls.amegroups.com/article/view/10.21037/ls-21-28/coif). The series “Laparoscopic Adrenalectomy” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical statement: The author is accountable for all aspects of the work including ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Lee J, El-Tamer M, Schifftner T, et al. Open and laparoscopic adrenalectomy: analysis of the National Surgical Quality Improvement Program. J Am Coll Surg 2008;206:953-9; discussion 959-61. [Crossref] [PubMed]
- Elfenbein DM, Scarborough JE, Speicher PJ, et al. Comparison of laparoscopic versus open adrenalectomy: results from American College of Surgeons-National Surgery Quality Improvement Project. J Surg Res 2013;184:216-20. [Crossref] [PubMed]
- Eichhorn-Wharry LI, Talpos GB, Rubinfeld I. Laparoscopic versus open adrenalectomy: another look at outcome using the Clavien classification system. Surgery 2012;152:1090-5. [Crossref] [PubMed]
- Walz MK, Peitgen K, Hoermann R, et al. Posterior retroperitoneoscopy as a new minimally invasive approach for adrenalectomy: results of 30 adrenalectomies in 27 patients. World J Surg 1996;20:769-74. [Crossref] [PubMed]
- Walz MK, Peitgen K, Walz MV, et al. Posterior retroperitoneoscopic adrenalectomy: lessons learned within five years. World J Surg 2001;25:728-34. [Crossref] [PubMed]
- Morino M, Benincà G, Giraudo G, et al. Robot-assisted vs laparoscopic adrenalectomy: a prospective randomized controlled trial. Surg Endosc 2004;18:1742-6. [Crossref] [PubMed]
- Brunaud L, Bresler L, Ayav A, et al. Robotic-assisted adrenalectomy: what advantages compared to lateral transperitoneal laparoscopic adrenalectomy? Am J Surg 2008;195:433-8. [Crossref] [PubMed]
- Winter JM, Talamini MA, Stanfield CL, et al. Thirty robotic adrenalectomies: a single institution's experience. Surg Endosc 2006;20:119-24. [Crossref] [PubMed]
- Patel N, Egan RJ, Carter BR, et al. Outcomes of surgery for benign and malignant adrenal disease from the British Association of Endocrine and Thyroid Surgeons' national registry. Br J Surg 2019;106:1495-503. [Crossref] [PubMed]
- Staubitz JI, Clerici T, Riss P, et al. EUROCRINE®: adrenal surgery 2015-2019- surprising initial results. Chirurg 2021;92:448-63. [Crossref] [PubMed]
- Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016;175:G1-G34. [Crossref] [PubMed]
- Fiori C, Checcucci E, Amparore D, et al. Adrenal tumours: open surgery versus minimally invasive surgery. Curr Opin Oncol 2020;32:27-34. [Crossref] [PubMed]
- Brix D, Allolio B, Fenske W, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol 2010;58:609-15. [Crossref] [PubMed]
- Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors Eur J Endocrinol 2018;179:G1-G46.
- Palazzo F, Dickinson A, Phillips B, et al. Adrenal surgery in England: better outcomes in high-volume practices. Clin Endocrinol (Oxf) 2016;85:17-20. [Crossref] [PubMed]
- Park HS, Roman SA, Sosa JA. Outcomes from 3144 adrenalectomies in the United States: which matters more, surgeon volume or specialty? Arch Surg 2009;144:1060-7. [Crossref] [PubMed]
- Goitein D, Mintz Y, Gross D, et al. Laparoscopic adrenalectomy: ascending the learning curve. Surg Endosc 2004;18:771-3. [Crossref] [PubMed]
- Guerrieri M, Campagnacci R, De Sanctis A, et al. The learning curve in laparoscopic adrenalectomy. J Endocrinol Invest 2008;31:531-6. [Crossref] [PubMed]
- Gimm O, Barczyński M, Mihai R, et al. Training in endocrine surgery. Langenbecks Arch Surg 2019;404:929-44. [Crossref] [PubMed]
- Hauch A, Al-Qurayshi Z, Kandil E. Factors associated with higher risk of complications after adrenal surgery. Ann Surg Oncol 2015;22:103-10. [Crossref] [PubMed]
- Gray WK, Day J, Briggs TWR, et al. Volume-outcome relationship for adrenalectomy: analysis of an administrative dataset for the Getting It Right First Time Programme. Br J Surg 2021;108:1112-9. [Crossref] [PubMed]
- Wass J, Lansdown M. Endocrinology GIRFT Programme National Specialty Report. 2021.
- Gaujoux S, Mihai R. joint working group of ESES and ENSAT. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg 2017;104:358-76. [Crossref] [PubMed]
- Griffin SB, Palmer MA, Strodl E, et al. Elective Surgery in Adult Patients with Excess Weight: Can Preoperative Dietary Interventions Improve Surgical Outcomes? A Systematic Review. Nutrients 2021;13:3775. [Crossref] [PubMed]
- Burnand KM, Lahiri RP, Burr N, et al. A randomised, single blinded trial, assessing the effect of a two week preoperative very low calorie diet on laparoscopic cholecystectomy in obese patients. HPB (Oxford) 2016;18:456-61. [Crossref] [PubMed]
- Fareau GG, Vassilopoulou-Sellin R. Hypercortisolemia and infection. Infect Dis Clin North Am 2007;21:639-57. viii. [Crossref] [PubMed]
- Boscaro M, Sonino N, Scarda A, et al. Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing's syndrome. J Clin Endocrinol Metab 2002;87:3662-6. [PubMed]
- Walz MK. Extent of adrenalectomy for adrenal neoplasm: cortical sparing (subtotal) versus total adrenalectomy. Surg Clin North Am 2004;84:743-53. [Crossref] [PubMed]
- Alesina PF, Hinrichs J, Meier B, et al. Minimally invasive cortical-sparing surgery for bilateral pheochromocytomas. Langenbecks Arch Surg 2012;397:233-8. [Crossref] [PubMed]
- Castinetti F, Taieb D, Henry JF, et al. MANAGEMENT OF ENDOCRINE DISEASE: Outcome of adrenal sparing surgery in heritable pheochromocytoma. Eur J Endocrinol 2016;174:R9-18. [Crossref] [PubMed]
- Puccini M, Panicucci E, Candalise V, et al. The role of laparoscopic resection of metastases to adrenal glands. Gland Surg 2017;6:350-4. [Crossref] [PubMed]
- Duh QY. Laparoscopic adrenalectomy for isolated adrenal metastasis: the right thing to do and the right way to do it. Ann Surg Oncol 2007;14:3288-9. [Crossref] [PubMed]
- Moreno P, de la Quintana Basarrate A, Musholt TJ, et al. Adrenalectomy for solid tumor metastases: results of a multicenter European study. Surgery 2013;154:1215-22; discussion 1222-3. [Crossref] [PubMed]
- Strong VE, D'Angelica M, Tang L, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol 2007;14:3392-400. [Crossref] [PubMed]
Cite this article as: Stechman MJ. Laparoscopic transperitoneal adrenalectomy—indications and technical aspects. Laparosc Surg 2022;6:14.


