
Endoscopic resection of GIST: feasible or fairytale?
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the GI tract, however they constitute less than 1% of all GI tumors.GISTs can be found in any segment of the alimentary tract, but most often are located in the stomach or small intestine which together represents about 90% of the localizations (Figure 1). Extra-GI involvement is very uncommon (1). The process that leads to the birth of these neoplasms originates from the interstitial cells of Cajal, which are located within the muscle layer of the GI tract, as discovered by Kindblom et al. back in 1998 (2). Approximately from 10% to 30% of GISTs have a malignant behaviour, however their classification is not based on this feature but they are rather stratified according to their clinical risk of malignancy: for example those lesions smaller than 20 mm with mitotic index less than 5 mitoses/50 high-power field (HPF) and without tumor rupture are considered at very low risk, while the other risk category (low risk, intermediate risk and high risk) vary according to size, mitotic index and primary tumor location or rupture (3). Primary GISTs can be symptomatic in up to 80% of cases, typically presenting with abdominal discomfort and obstructive symptoms or even gastrointestinal bleeding. Incidental discovery of asymptomatic GISTs occurs in less than 20% of cases, commonly during upper or lower GI endoscopy or others radiologic investigation. The diagnostic work-up is essentially based on gastrointestinal endoscopy also with endoscopic ultrasound (EUS) scan, magnetic resonance imaging (MRI) scan, computed tomography (CT) scan and 18fluoro-deoxyglucose-positron emission tomography (18FDG-PET) scan. When feasible, depending by their size and location, histological confirmation can be obtained with EUS scan by fine needle aspiration biopsy (4). As reported by several national guidelines, complete surgical resection (R0) is the gold standard treatment for localized GIST: segmental resection of intestine and wedge resection of stomach are commonly accepted. This is particularly true when we consider GISTs lesions larger than 2 cm in size, with more than 5 mitoses/50 HPF which carry significant risk of lymph node metastasis and recurrence; this type of neoplasm therefore should be considered for surgical resection. Also the National Comprehensive Cancer Network (NCCN) and the American Society for Gastrointestinal Endoscopy (ASGE) recommend resection of GISTs that are clinically relevant (>2 cm) or have a histological evidence of being malignant lesions, and those neoplasms with high-risk features on EUS or the one in which there has been a rapid increase in size: for this kind of tumors the standard of care procedure is their complete surgical resection with sufficient surgical margins (5-7). Crucial point when operating these neoplasms with a curative intent, is to avoid tumor rupture as it is associated with high-risk of sarcomatosis which significantly worsens the overall patient’s survival (5,8). Having said that, a growing body of evidence seems to confirm that GISTs smaller than 2 cm in size with less than 5 mitoses/50 HPF, can be endoscopically resected safely. Recently with the development of endoscopy techniques and instruments, new endoscopic possibilities have been introduced in clinical practice, namely endoscopic full-thickness resection (EFTR) and submucosal tunneling endoscopic resection (STER).
Endoscopic technique
EFTR
EFTR is a technique that guarantees minimum invasiveness for en bloc resection of gastrointestinal lesions (Figure 2); thus, it provides for attainment of a complete tumor specimen for pathological study. The first described EFTR was reported by Suzuki and Ikeda, back in 1998. Three years later in 2001, the same authors have verified its effectiveness in treating submucosal tumors which include, among others, GISTs (9). In order to achieve a complete resection of the target lesion, EFTR procedure deliberately causes a continuous solution within the GI wall. It is then of paramount importance to close the GI wall defect in an effective and safe manner. There are two different EFTR techniques: the first one is called “free-hands” in which after completing a full thickness excision of the neoplasm, the wall defect suture is carried out; the second one is known as “device-assisted” during which both the resection and the patency of the GI tract is secured by the same clip/device deployment (Figure 3) (10). The crucial steps of the procedure are summarized in Figure 4.
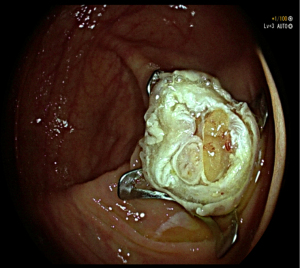
In 2011, Zhou et al. reported 26 EFTR for submucosal tumors (SMTs) originated from the muscularis propria. Pathological diagnosis of these lesions included GISTs (16/26), leiomyomas (6/26), glomus tumors (3/26), and Schwannoma (1/26). Complete resection was obtained in all tumors, with a median size slightly less than 30 mm, ranging from 12 to 45 mm; more importantly no lesion residual or recurrence was observed during the surveillance period. Resected opening of full thickness layer was successfully closed by Endoclips in all cases (11). More recently, Guo et al. included in their study 23 patients with gastric tumors originating from the muscularis propria and treated with EFTR. The full-thickness resection rate was 100% (23/23), with no residual tumor at the cutting edge, as documented by pathological diagnosis; likewise, the success rate of defect closure was 100% thanks to the over-the-scope clip (OTCS) system. Pathology report confirmed that 83% (19/23) of neoplasms were GIST, while the other 4 were leiomyomas (12). In 2017, Yu et al. reported the first data on the long-term outcomes of resection of gastric GISTs with an endoscopic technique. In their study 60 patients were treated with a follow-up period of 36.15±12.92 months. In this retrospective series only one patient suffered from primary tumor recurrence, while the others showed no recurrence, metastasis, or death. The authors concluded that endoscopic resection of gastric GISTs is a safe and reliable approach, with satisfying outcomes in the long-term; however, it should be reserved for small gastric GISTs (no more than 5 cm in size) without metastasis or high risk features (13). A systematic review concerning the role of EFTR in gastric submucosal tumor (including 112 GISTs) was published by Jain and colleagues in 2016. In this review, regarding to EFTR of stomach tumors originating from muscular layer, the mean success rate was 96.8%, defined as a complete resection of tumor with clear margins microscopically. During the follow-up visits no recurrence were reported; however, the were no consensus on follow up protocol after EFTR of gastric submucosal tumors (SMTs) (14). Interestingly, Wang et al. carried out during 2015 a comparison study of the efficacy and safety of EFTR and laparoscopic-assisted surgery for small (<2 cm) GISTs. In this research 68 subjects with GISTs originating from the muscularis propria were included: 35 of them were treated endoscopically while the other 33 with conventional surgery. All enrolled subjects were monitored up to 72 months and no local recurrence were observed. Not surprisingly, in the EFTR group procedure time was significantly shorter than that in the laparoscopic-assisted surgery group, as well as hospital stay and time of food intake resume. To be noticed, the complication rate in the endoscopic group was significantly lower compared to the one in surgery group. The authors therefore state that EFTR is a secure treatment alternative, providing excellent pathological diagnosis for GISTs lesser than 2 cm in size (15).
STER
STER, also known as SET (subepithelial tumor resection) or POET (per-oral endoscopic tumor resection), is a technique in which a submucosal tunnel is established to serve as a working space for the insertion of endoscopic tools and subsequent removal of the target lesion. It was inspired by endoscopic submucosal dissection (ESD) and it is a natural evolution of the endoscopic tunneling technique (e.g., per-oral endoscopic myotomy or POEM). One of the advantages of STER compared to other procedure, is the relative easiness of closure tunnel compared, for example, to the full-thickness wall defect created during EFTR. The most suitable structures for STER include those organs in which the lumen is straight and tubular shaped, such as the oesophagus or gastric cardia, in order to create a submucosal space enough large enough to safely remove the neoplasm (16). However, STER can be applied in locations such as the gastric cavity and lower GI tract (particularly in the rectum) without a significant raise in adverse events. Nevertheless, there are some contraindications to the procedure: STER should not be taken into account in case of ulcerated mucosa, when the lesion has irregular borders and when a deep portion of the muscularis propria is involved (17). The fundamental steps of the procedure are reported in Figure 5.
A huge meta-analysis on this technique was performed by Lv et al. during 2017. The authors included in their paper 28 studies conducted between 2011 and 2015, with more than 1,000 patients treated with STER for upper gastrointestinal SMTs. The pooled complete resection and en bloc resection rates were 97.5% and 94.6%, respectively. There was a low rate of adverse events, being air leakage symptoms the most common adverse event, with a pooled estimate rate of 14.8%. No local recurrence was found in any of the studies taken into account during the surveillance periods, although there are no guidelines regarding ideal follow up; moreover, no STER-related deaths occurred (18). A retrospective study with a more homogeneous follow-up was carried out by Chan and associates. A total of 180 patients undergoing STER for SMTs located in the upper gastrointestinal tract have been monitored with a median follow-up of 36 months. During the surveillance period no local recurrence or distant metastasis were reported. En bloc resection was achieved in the large majority of patients (>90%) with a low complication rate (8.3%). The authors conclude that STER is a feasible and effective treatment option, in particular for tumors located in the oesophagus and cardia with a long diameter ≤5.0 cm and a transverse diameter ≤3.5 cm (19).
Comparison between techniques: EFTR vs. STER
Tan et al. retrospectively collected between 2011 and 2016 all clinical data of patients with gastric GISTs treated with one of the two techniques mentioned, STER or EFTR. A total of 52 patients were enrolled (20 STER and 32 EFTR). Among the two cohorts no statistically significant differences were found in terms of demographic characteristics, tumor dimension, operation time, length of hospitalization and overall procedure cost. However, STER group had a shorter suture time and required fewer clips for closure of the wall defect; this finding is likely to be linked to the intrinsic characteristics of the two procedures, as previously mentioned. En bloc resection was achieved in more than 95% of patients, with no statistically significant differences. In terms of complications, no significant difference between the two groups was found. Moreover, no recurrence was detected in both groups during a mean follow-up of about 11 months for STER and almost 2 years for EFTR (20). Another collection of retrospective data comparing the two techniques was carried out by Duan and colleagues in 2017. In this study 43 patients with lesions located in the gastric cavity were enrolled. After the pathological evaluation the removed neoplasms were found to be GIST in 36 cases, 6 were leiomyoma and 1 was a schwannoma instead. 34.9% (15/43) of the cases underwent STER and the remaining 28 (65.1%) underwent EFTR. As also reported in the previous mentioned study, no significant differences were noted between the two groups in terms of age, gender, concurrent pathologies, tumor size, operation time and overall cost. Even in this case suturing time and postoperative hospital stay was shorter in the STER group. Authors confirmed the high rate of en bloc resection: 93.3% of the subjects treated with STER and 96.4% of the one who underwent EFTR respectively, with no statistically significant difference. The rate of adverse events was not different between the two treatments and no recurrence was noted in both groups (21).
Conclusion
Despite being at the boundaries of therapeutic endoscopy, EFTR and STER seem to be safe, feasible and effective procedures to treat those GISTs in which conventional surgery would be too demolitive. Something important that must be stressed is that STER and EFTR are largely not coinciding in terms of anatomical location, being STER preferable for oesophageal or cardial neoplasms, where tunneling is easier to perform; on the other hand, EFTR may be a more recommendable choice for those lesions of irregular shape and larger dimensions located in the gastric cavity. In addition, due to relative rarity and heterogeneity of this pathology, individualization is mandatory: careful selection of candidates by preoperative EUS/CT evaluation to exclude neoplasms that have already shown their malignant potential and to confirm the size and location of lesion remains crucial. Although the data present in the literature are promising, there are still some unclear points that need to be clarified, such as the long-term outcomes and the reproducibility of the described techniques. Other studies, possibly including larger populations and with prospective and randomized designs, are therefore necessary to fully understand the real potential of these approaches.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Cananzi Ferdinando Hunimed) for the series “Minimally invasive approach in Gastrointestinal Stromal Tumors (GISTs)” published in Laparoscopic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ls.amegroups.com/article/view/10.21037/ls-20-136/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ls.amegroups.com/article/view/10.21037/ls-20-136/coif). The series “Minimally invasive approach in Gastrointestinal Stromal Tumors (GISTs)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Judson I, Demetri G. Advances in the treatment of gastrointestinal stromal tumours. Ann Oncol 2007;18:x20-4. [Crossref] [PubMed]
- Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Cho JWKorean ESD Study Group. Current Guidelines in the Management of Upper Gastrointestinal Subepithelial Tumors. Clin Endosc 2016;49:235-40. [Crossref] [PubMed]
- Nishida T, Goto O, Raut CP, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016;122:3110-8. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8:S1-41; quiz S42-4. [Crossref] [PubMed]
- Landi B, Blay JY, Bonvalot S, et al. Gastrointestinal stromal tumours (GISTs): French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis 2019;51:1223-31. [Crossref] [PubMed]
- Poveda A, García Del Muro X, López-Guerrero JA, et al. GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treat Rev 2017;55:107-19. [Crossref] [PubMed]
- Suzuki H, Ikeda K. Endoscopic mucosal resection and full thickness resection with complete defect closure for early gastrointestinal malignancies. Endoscopy 2001;33:437-9. [Crossref] [PubMed]
- Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc 2018;30:17-24. [Crossref] [PubMed]
- Zhou PH, Yao LQ, Qin XY, et al. Endoscopic full‐thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 2011;25:2926-31. [Crossref] [PubMed]
- Guo J, Liu Z, Sun S, et al. Endoscopic full‐thickness resection with defect closure using an over‐the‐scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc 2015;29:3356-62. [Crossref] [PubMed]
- Yu C, Liao G, Fan C, et al. Long-term outcomes of endoscopic resection of gastric GISTs. Surg Endosc 2017;31:4799-804. [Crossref] [PubMed]
- Jain D, Mahmood E, Desai A, et al. Endoscopic full thickness resection for gastric tumors originating from muscularis propria. World J Gastrointest Endosc 2016;8:489-95. [Crossref] [PubMed]
- Wang H, Feng X, Ye S, et al. A comparison of the efficacy and safety of endoscopic full-thickness resection and laparoscopic-assisted surgery for small gastrointestinal stromal tumors. Surg Endosc 2016;30:3357-61. [Crossref] [PubMed]
- Dellatore P, Bhagat V, Kahaleh M. Endoscopic full thickness resection versus sub-mucosal tunneling endoscopic resection for removal of submucosal tumors: a review article. Transl Gastroenterol Hepatol 2019;4:45. [Crossref] [PubMed]
- Rajan E, Wong Kee Song LM. Endoscopic Full Thickness Resection. Gastroenterology 2018;154:1925-1937.e2. [Crossref] [PubMed]
- Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and me-ta-analysis. Surg Endosc 2017;31:49-63. [Crossref] [PubMed]
- Chen T, Zhou PH, Chu Y, et al. Long-term Outcomes of Submucosal Tunneling Endoscopic Resection for Upper Gastrointestinal Submucosal Tumors. Ann Surg 2017;265:363-9. [Crossref] [PubMed]
- Tan Y, Tang X, Guo T, et al. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc 2017;31:3376-82. [Crossref] [PubMed]
- Duan TY, Tan YY, Wang XH, et al. A comparison of submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric fundus submucosal tumors. Rev Esp Enferm Dig 2018;110:160-5. [PubMed]
Cite this article as: Colombo M, Spadaccini M, Maselli R. Endoscopic resection of GIST: feasible or fairytale? Laparosc Surg 2022;6:12.






