
Laparoscopic thermal ablation for colorectal liver metastases: technical tips and pitfalls
Introduction
Actually, liver resection is the standard of care for treatment of patients affected by liver metastases from primary colorectal cancer (CRLMs), ensuring remarkable 5-year survival rates (up to 58%) with low mortality (1–3%) (1). Nevertheless, only a small group of them (15–20%) are amenable to upfront liver resection. In the last two decades, the sinergy among surgeons and oncologists, thus applying technologically advanced procedures [portal embolization, local ablations techniques, two stage hepatectomy and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS)], with new anti-angiogenic agents, significantly impacted on rate of resectable patients up to 30% (2).
The loco-regional treatments focused on liver tumors may be classified into those using either chemicals or extreme temperatures. The first group was mostly represented by percutaneous ethanol injection (PEI), while the second one consists of therapies employing both very low temperatures (cryoablation) and very high temperatures achieved either by electromagnetic waves, such as radiofrequency (RFA), microwave (MWA) and light waves (laser) ablations, or by sound waves, such as high-intensity focused ultrasound (HIFU). RFA and MWA are currently the most widely diffused and effective interstitial ablative technique (3). In the first era, since 1990’s RFA had a worldwide diffusion due its efficacy and low morbidity (4,5). More recently, while RFA technology development didn’t move forward, MWA was expanding rapidly thus gaining its success due to its more homogeneous and powerful thermal effect (6). In the actual scenario of minimally invasive liver surgery, laparoscopic thermal ablation (LTA) gained a huge expansion within multimodal treatment of CRLMs, either alone for small, deep and tricky lesions not visible at percutaneous US, or more frequently adjuvant to resection, within a one or two step operation especially for bilobar CRLMs (7). Our aim is to review the current role of laparoscopic ablative approach in the management of CRLMs. In the first part of the paper specific considerations concern Laparoscopic via will be explained, while in the second one crucial open issues regard TA will be held.
Methods
We conducted a PubMed research to select papers of interest. The following keywords were used to search in title or abstracts: “colorectal liver metastases” and “laparoscopic thermal ablation” or “laparoscopic radiofrequency ablation” or RFA” or “minimally invasive radiofrequency ablation” or “microwave ablation” or “MWA”. Our research was restricted to English articles published from February 1990 to February 2022 and for which full text was achievable.
LTAs for the treatment of CRLMs
Indications for LTA
The literature regarding indication for TA for patients with resectable CRLMs is quite exclusively based on retrospective studies, often limited by the iniquity of baseline line of comparative group, thus actually TA (regardless the type of approach) should not be used instead of surgical resection, as reported in COLLISION Trial (8); moreover, the choice of percutaneous vs. surgical via, depends on the specific strategy decided in a multidisciplinary setting.
Over the last two decades, TA has been incorporated into the treatment of unfit for surgery patients with CRLMs (9). Following are the most common indications for TA for the treatment of CRLMs reported in literature regardless of approach:
- Adjuvant to resection in the presence of not enough functional remnant liver volume or persistent nodule in the residual liver (7,10-13);
- Fit for surgery but small lesion (<30 mm) solitary and deep nodule, instead of a non-acceptable huge resection, after discussion of pro and cons (14-17);
- Patients unsuitable for hepatectomy with less than 8 CRLMs and largest lesion less than 40 mm (8);
- Unfit for surgery for elderly people, performance status, comorbidities and/or personal refusal (18);
In addition, there are specific indications for LTA for CRLMs in the event of the following “tricky” scenarios (7,19-21):
- Patients with nodules not visible at percutaneous US:
- Lesions in the liver dome about the diaphragm;
- Lesions in posterosuperior segments;
- Lesions in sub-glissonian position;
- Lesions next to stomach, duodenum, right colonic flexure or kidney;
- Lesions next to gallbladder.
- Patients under severe coagulation’s disorders;
- In combination with surgery within a minimally invasive two-stage hepatectomy/ALPPS strategy;
These indications are indicative and effective for primary and recurrent disease and should always be determined by multidisciplinary evaluation. We have to keep in mind that progression after systemic treatment and/or extrahepatic disease are an oncological contraindication for TA (7).
LTA: technical tips and tricks
There are three methods to perform TA: percutaneous, open and laparoscopic approach. Instead of for what concern the treatment of hepatocellular carcinoma in which, the percutaneous TA is the favorite used in almost 75% of cases (22), surgical TA, is widely diffused for the treatment of CRLMs (23), preferable in combination with surgical resection. PTA has several limitations, including potential higher local tumor recurrence (LTR) rate compared to surgical via, inferior tumor staging and limitations to treat superficial or tricky located metastases (24,25).
Mimmo et al. in a recent systematic review analyzed 12 studies concerning patients submitted to MWA +/− surgery for resectable CRLMs, reporting TA was done in majority of cases trough surgical approach (85.9%) of whom 47.5% via laparoscopy (26). Surgical TA was chosen for multiple (72.6%) and synchronous (55.9%) lesions, thus reporting vs. the percutaneous via, the same morbidity (26.8% vs. 25%) and a better free from LTR rate at 12 months (88.6% vs. 78.5%). These results reflect effectiveness of the surgical TA applied to patients with higher disease’s burden.
Even if LTA requires general anesthesia and high skill in laparoscopic ultrasound (LUS), it pooled advantages of both open and percutaneous via (27,28). LTA showed better results in terms of LTR due to better targeting for tricky located nodules and a more aggressive ablation thanks to ongoing US monitoring (9,25). Moreover, LUS may safely identify further small nodules in the liver or peritoneal carcinosis, not seen on preoperative imaging studies (29-32).
As reported above, LTA alone is quite a rare option, which can be requested to treat CRLMs unfit either for open surgery, percutaneous TA or for chemotherapy. First of all, patient’s position in operative room is crucial to get easier the targeting of the CRLMs. As previously described for hepatocellular carcinoma (HCC) (33), there different positions of the patients depending on site of the nodule, with the crucial issue the target has to be always on top of the surgeon’s viewing (Figure 1).
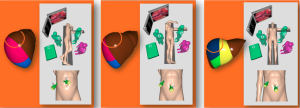
The more lesion is going towards right posterior segments, the more the patient will be tilted trough a left “thoracotomy” position. Patients generally lie supine with close legs, but in front of previous surgery with expected adhesions, abduction of thighs is recommended with surgeon among them. First step is always intraoperative LUS examination with a dedicated probe (7.5 linear array transducer) and a systematic technique previously described in literature (34). Since laparoscopic approach is often performed for tricky lesions, in selected cases the following technical tips are suggested (33):
- “Water technique” for lesions in the liver dome: Trendelenburg position and introduction of saline solution in the space between liver and diaphragm, thus optimizing US acoustic window to target CRLM;
- “Separating technique” for lesions adjacent to abdominal viscera: after targeting the liver metastases, a further 5 mm laparoscopic access is required to separate target from viscera by using a atraumatic grasping forceps;
- “Cooling technique” for the same case of type II, by perfusing the space between liver and viscera to preserve with cold saline solution during all the procedure.
Moreover, LTA can be used in combination with surgery especially as a first step of a two stage hepatectomy to treat small nodule in the future remnant liver or to draw a sharp transection line during the first phase of ALPPS, thus providing either minimum adhesion formation thanks to less aggressive procedure or bleeding after first stage due to the pneumoperitoneum and perfect visibility of surgical field due to magnification of high resolution view (35). Moreover, advantages of LTA within TSH, were observed in terms of lower hospital stay especially in the first stage (4 vs. 7.5 days, P<0.001) and earlier administration of chemotherapy after second stage (1.6 vs. 2 months, P=0.039) (36). This minimally invasive two stages surgical (resection +/− thermal ablation) approach proved to be feasible with a completion rate of 80% of cases and effective thus optimizing timing for chemotherapy. Nevertheless, either publications or experiences in the laparoscopic approach to TSH have remained limited due its complexity requiring a multidisciplinary team in expert centers (37).
In 2016, Jiao firstly introduced LTA as a tool to increase the future liver remnant, modifying ALPPS in RALPP, that is radiofrequency assisted liver partition with portal vein ligation (RALPP), where surgical portal vein ligation and TA of the parenchyma is performed without splitting liver parenchyma to limit complications related to ALPPS (38).
Hot topics beyond the type of TA approach in patients with CRLMs
RFA vs. MWA, which system runs better
There are mainly two TA systems involved in the treatment of CRLMs: RFA and MWA. Both of them apply thermal injury to the tumor thus destroying it and the narrowing tissue. Nevertheless, these two systems have been introduced by years, their application have not been so widely diffused in the field of CRLMs compared to HCC for many reasons. First of all, both under layer liver and shape of tumor in CRLMs setting are quite different thus conditioning a lower and less effective distribution of thermic wave due to lack of “oven effect” (39). This theory initially described by Liu et al. is based on the fact cirrhotic parenchyma works as a refractive tissue that focus energy thus allowing higher temperatures and more homogeneous ablation areas (39,40). The normal liver parenchyma is generally smooth and the metastases not capsulated, thus heat diffusion within the tumor is less homogeneous and effective compared to HCC in cirrhotic liver (39). Nevertheless, this intrinsic limit, both technologies of TA have been shown to be effective in the treatment of patients with small CRLMs. Either laparoscopic needle of RFA or antenna of MWA has different length ranging between 25 and 30 cm in order to reach under pneumoperitoneum, distal and tricky located lesions for example ones abutting the diaphragm. RFA uses a form of alternating electrical current at a frequency of 400 MHz to generate thermal energy via ionic agitation thus causing ovoid necrosis of the tissue next to the tip of the needle (23). MWA uses electromagnetic waves to agitate water and generate heat for tumor destruction using devices with frequencies greater than 900 MHz (6).
Since its introduction in the late 90’s, RFA gained a high diffusion worldwide to treat patients with small unresectable CLRMs, due to its safety and easy reproducibility (41). The introduction of microwaves technology in the last decade drastically changed this paradigm, due to multiple MWA’s advantages compared to RFA for the treatment of CRLMs, as summarized below:
- Larger and more predictable, spherical and homogenous area of necrosis (42);
- Higher intratumoral temperatures (43,44);
- Faster ablation times (43);
- No “heat sink effect” (45);
- Major investments in technology.
Recent developments of new MWA technologies with the employ of higher frequency bands (2,46), surface energy control, and “cooled tip” antenna (technology consisted in saline irrigation channels within the tip, developed also for RFA), helped to obtain more homogenous, effective and predictable heat effect.
For what concern perioperative results, it’s crucial to underline there are bias due to data coming from often not comparable papers where thermal ablation was used to treat patients unfit for surgery or in combination with it. Both systems (RFA and MWA) have been shown to be safe thus showing low morbidity and mortality mildly in favor of RFA, respectively 6–9% vs. 0–54% and 1 vs. 0–2%. By comparing MWA to RFA, the former showed a lower LTR rates vs. RFA (6% vs. 20%), while a worse 5-year OS rate (18% vs. 25–55%) (14,16,47).
The size of the target: which ideal cut-off
Among multiple parameters conditioning the choice of TA, the size of the target constitutes the most important issue, thus being directly responsible for recurrence within the ablation area (LTR) (25). We’ll speak about maximum acceptable diameter of the target by preferably using MWA for its ability to create wider areas of necrosis. The goal of liver MWA for CRLMs is to achieve an area of necrosis overcoming both tumor and at least 10 mm rim of surrounding liver parenchyma. The majority of patients (up to 87%) usually received chemotherapy prior the thermal ablation (48), and it has been described that rate of viable micro-metastases surrounding the target varies widely, ranging from 31% to 70% (49). Wakai et al. evaluated incidence and distribution of micrometastases surrounding the target for patients submitted to liver resections with or without neoadjuvant chemotherapy, showing distance from the advancing border of the CRLMs to each intrahepatic micrometastases ranged between 0.1 and 17 mm (median 2 mm) and in particular <10 mm in 98% of patients with prior chemotherapy (49). Since several papers regarding both TA or resection, reported the mean maximal ablation diameter is 5.5 cm (25,50), and it’s widely accepted the ablation zone ideally contains a rim of normal liver tissue approximately 1 cm beyond a tumor in each dimension, tumor 3 cm or less will be the ideal cut off of current microwave technology (27,44,51). According to this crucial issue, Benhaim in his series of 83 patients submitted to TA, reported a higher LTR at 1-year, in group of patients with <25 mm tumor size shrinkage after chemotherapy (before chemo >25 mm), versus group with <25 mm up front (32% vs. 16%, P≤0.001) (52).
Nielsen reported LTR following TA of CRLMs in 39 out of 128 patients (30.4%), and this percentage was directly related on target’s size: 0–3 cm, 9%, 3–5 cm (26.5%), and >5 cm; 45% (44). In a recent series of sixty-one consecutive patients affected by CRLMs submitted to TA via either open or laparoscopic approach, LTR occurred in 11% of lesions <20 mm vs. 50% >20 mm (P=0.009) (7). Alksoy reported LTR in 118 patients (47%) out of 225 submitted to laparoscopic thermal ablation for CRLMs with a dominant tumor size of 29 mm (range, 7–65 mm) (53). Tumor size and ablation margin resulted independent predictors of LTR: nodules >5 cm had twice the risk of LTR vs. those <3 cm and nodules ablated with a <1-cm margin had a 1.9-fold increased risk of LTR vs. tumors with a >2-cm margin (53). Moreover, Mulier et al. in his recent review including 5,224 CRLMs submitted to TA, reported a huge variation of LTR rate (range 2–60%), thus showing a significant advantage in term of LTR for small size (P<0.001) and laparoscopic vs. percutaneous approach (P<0.001) (25,54).
Ablation therapies vs. surgical resection, is it always an impair match?
What does it mean to compare TA vs. resection? In order, not to match apples to pears, we have to know that until now, there has been only one RCT comparing RFA to surgery for CRLMs in the same setting (14), while most of the reported studies in literature are nonrandomized and retrospective papers including following scenarios (55-57):
- TA for unresectable CRLMs vs. resection (1,13,18,21,48,58-64);
- TA for resectable CRLMs vs. resection (7,14-17);
- TA for resectable CRLMs vs. resection when TA unfeasible (16);
- TA plus resection in almost unresectable patients vs. resection (10-13,39);
- TA plus resection in one stage hepatectomy (TSH) for bilobar CRLMs vs. TSH without TA (65).
At regards a recent systematic review and meta-analysis based on 22 studies concerning patients submitted to TA vs. surgery for CRLMs (55) showed no difference in terms of 30-day mortality among procedures, but a worse performance of TA for what concern LTR, 5-year survival and DFS rate. Nevertheless, authors underlined these results were limited to the iniquity of baseline line of comparative group, thus as a matter of fact, in most papers currently available in literature, the patients submitted to TA were unresectable and had a worse clinicopathologic features than resectable patients undergoing liver resection (extrahepatic disease, higher tumor burden and more comorbidities).
Anyway, there are seventeen studies comparing TA vs. surgery in resectable patients with CRLMs, of whom 3 via laparoscopic approach (15,18,48) (Table 1). Thirteen out of those (76.5%), considered TA when surgical approach was unfeasible for technical reasons, comorbidities, patient decision or intraoperative findings (1,13,18,21,48,57-64), thus is easily predictable the superiority of resection arm over TA especially in terms of LTR. Minor gap has been reported about overall survival rate for several reasons:
Table 1
| Author | Year of publication | Study | Patients with CRLMs (LAT/RES) | Type of approach | Criteria for LAT | Median size of tumors for TA (cm) |
LTR: TA vs. RES (%) | P value | 5-year OS: TA vs. RES (%) | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Van de Geest (17) | 2022 | RO, PSM | 72 (36/36) | O | R | 2.5 | 24 vs. 13 | 0.73 | 42 vs. 53 | 0.09 |
| Huang (57) | 2021 | RO, PSM | 184 (98/86) | O | UR | NR | NR | NR | 44.5 vs. 50.8 | 0.943 |
| Hof (61) | 2016 | RO | 362 (101/261) | O | UR | 2.2 | 26.9 vs. NR | NR | 53 vs. 51.9 | 0.979 |
| Jasarovic (64) | 2014 | RO | 140 (46/94) | O | UR | 3.8 | 34 vs. 12.8 | 0.026 | NR | NR |
| Aliyev (18) | 2013 | RO | 104 (44/60) | L | UR | 2.3 | 18 vs. 4 | 0.012 | 47/57 | 0.464 |
| Agcaouglu (48) | 2013 | RO | 389 (94/295) | L | UR | 3.4 | 45 vs. 9 | NR | 17/58 | 0.001 |
| Kim (59) | 2011 | RO | 455 (177/278) | O | UR | 2.1 | NR | NR | 51.2 vs. 51.1 | 0.96 |
| Lee (21) | 2011 | RO | 53 (28/25) | O | UR | 2.05 | 42.9 vs. 8 | 0.004 | 24 vs. 41* | 0.017 |
| Hur (58) | 2009 | RO | 67 (25/42) | O | UR | 2.5 | 28 vs. 9.5 | 0.085 | 25.5 vs. 50.1 | 0.0263 |
| Otto (16) | 2010 | RO | 110 (28/82) | O | R | 3 | 32 vs. 4 | <0.001 | 48 vs. 51 | 0.961 |
| McKay (63) | 2009 | RO | 101 (43/58) | O | UR | 3 | 60 vs. 7 | <0.0005 | 23 vs. 43 | 0.02 |
| Gleisner (13) | 2008 | RO | 203 (11/192) | O | UR | 3 | 41.3 vs. 2 | <0.001 | 72.7 vs. 74.1 | 0.50 |
| Berber (15) | 2008 | RO | 158 (68/90) | L | R | 3.7 | 16 vs. 2 | NR | 30 vs. 40 | 0.35 |
| Aloia (62) | 2006 | RO | 180 (30/150) | O | UR | 3 | 37 vs. 5 | <0.001 | 27 vs. 71 | <0.001 |
| Abdalla (1) | 2004 | RO | 247 (57/190) | O | UR | 2.5 | 9 vs. 2 | 0.02 | 21 vs. 58 | <0.0001 |
| Oshovo (60) | 2003 | RO | 45 (25/20) | O | UR | 3 | NR | NR | 52.6 vs. 55.4 | NR |
| Shibata (14) | 2000 | RPT | 30 (14/16) | O | R | 2.7 | NR | 14 vs. 23** | 0.083 |
*, 4-year survival; **, 3-year survival. TA, thermal ablation; CRLMs, colorectal liver metastases; RES, resection; OS, overall survival; RO, retrospective observational; RPT, randomized prospective trial; PSM, propensity score matching; LTR, local tumor recurrence; O, open; L, laparoscopic; R, resectable; UR, unresectable; NR, not reported.
- Different tumor biologic profiles;
- Multiple lines of chemotherapy;
- Chance of re-treatment.
There are only four papers concerning TA versus resection for resectable CRLMs, of whom three retrospective observational studies and only one randomized prospective series, dating back to 2000 (9,14,16,17). Among three observational studies, only Van de Geest used propensity match score to reduce influence of known covariates such as age, comorbidities, and tumor features (17). In these retrospective papers, majority of patients had multiple lesions, with mean target diameter of 3 cm, and it was confirmed a higher rate of LTR in TA vs. resection group respectively of 24% vs. 13% (P=0.73) (17), 32% vs. 4% (P<0.001) (16) and 16% vs. 2% (15). Shibata in his prospective series on 30 patients randomized submitted to TA (n=14) vs. resection (n=16) for resectable multiple CRLMs, with median target diameter of 2.7 cm, suggested TA to be equally effective as hepatic resection in terms of 1-, 2- and 3-year survival rates (14% vs. 23%, P=0.003) (14).
In conclusion, many studies definitively reported that hepatectomy plus TA for otherwise unresectable patients can achieve OS and hepatic recurrence free survival that is similar to the hepatectomy alone in terms of effectiveness and safety (56).
On the contrary, in the setting of resectable CRLMs, actually high evidence based studies matching TA and liver surgery for CRLMa are still lacking. At regards, a recently published a phase III single-blind prospective randomized controlled trial, called “COLLISION”, has been drawn to prove non-inferiority of thermal ablation compared to liver resection in patients with resectable ≤3 cm CRLMs (8). The whole duration of the study, began in 2017, will be 13 years, with an inclusion time of 3 years and a follow up of 3 years. Until then, to current-day, it should be unethical to consider TA as curative option rather than surgery when feasible therefore, the highest achievable evidence level for unresectable CRLM seems to be reached for the time being (66).
Conclusions
LTA is an effective and safe procedure alone or in combination with surgery for the treatment of CRLMs. Randomized prospective studies comparing TA vs. resection for patients affected by resectable CRLMs are requested to define the role of local ablative therapies in this crucial setting, but for the moment, TA seems to remains adjuvant to surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Santambrogio and Marco Antonio Zappa) for the series “Laparoscopic Hepato-Biliary Surgery” published in Laparoscopic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ls.amegroups.com/article/view/10.21037/ls-22-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ls.amegroups.com/article/view/10.21037/ls-22-12/coif). The series “Laparoscopic Hepato-Biliary Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7. [Crossref] [PubMed]
- Cirocchi R, Trastulli S, Boselli C, et al. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev 2012;CD006317. [Crossref] [PubMed]
- Cunningham SC, Choti MA, Bellavance EC, et al. Palliation of hepatic tumors. Surg Oncol 2007;16:277-91. [Crossref] [PubMed]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575-80. [Crossref] [PubMed]
- Korpan NN. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg 1997;225:193-201. [Crossref] [PubMed]
- Martin RC, Scoggins CR, McMasters KM. Microwave hepatic ablation: initial experience of safety and efficacy. J Surg Oncol 2007;96:481-6. [Crossref] [PubMed]
- Barabino M, Gatti A, Santambrogio R, et al. Intraoperative Local Ablative Therapies Combined with Surgery for the Treatment of Bilobar Colorectal Liver Metastases. Anticancer Res 2017;37:2743-50. [Crossref] [PubMed]
- Puijk RS, Ruarus AH, Vroomen LGPH, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 2018;18:821. [Crossref] [PubMed]
- Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol 2008;15:2757-64. [Crossref] [PubMed]
- Eltawil KM, Boame N, Mimeault R, et al. Patterns of recurrence following selective intraoperative radiofrequency ablation as an adjunct to hepatic resection for colorectal liver metastases. J Surg Oncol 2014;110:734-8. [Crossref] [PubMed]
- Engstrand J, Nilsson H, Jansson A, et al. A multiple microwave ablation strategy in patients with initially unresectable colorectal cancer liver metastases - A safety and feasibility study of a new concept. Eur J Surg Oncol 2014;40:1488-93. [Crossref] [PubMed]
- Karanicolas PJ, Jarnagin WR, Gonen M, et al. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surg 2013;148:597-601. [Crossref] [PubMed]
- Gleisner AL, Choti MA, Assumpcao L, et al. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 2008;143:1204-12. [Crossref] [PubMed]
- Shibata T, Niinobu T, Ogata N, et al. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 2000;89:276-84. [Crossref] [PubMed]
- Berber E, Flesher N, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg 2002;26:985-90. [Crossref] [PubMed]
- Otto G, Düber C, Hoppe-Lotichius M, et al. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg 2010;251:796-803. [Crossref] [PubMed]
- van de Geest TW, van Amerongen MJ, Nierop PMH, et al. Propensity score matching demonstrates similar results for radiofrequency ablation compared to surgical resection in colorectal liver metastases. Eur J Surg Oncol 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Aliyev S, Agcaoglu O, Aksoy E, et al. Efficacy of laparoscopic radiofrequency ablation for the treatment of patients with small solitary colorectal liver metastasis. Surgery 2013;154:556-62. [Crossref] [PubMed]
- Giglio MC, Logghe B, Garofalo E, et al. Laparoscopic Versus Open Thermal Ablation of Colorectal Liver Metastases: A Propensity Score-Based Analysis of Local Control of the Ablated Tumors. Ann Surg Oncol 2020;27:2370-80. [Crossref] [PubMed]
- Santambrogio R, Barabino M, Galfrascoli E, et al. Laparoscopic radiofrequency of liver metastasis: is its cost effectiveness a good reason to replace hepatic resection? Laparosc Surg 2019;3:20. [Crossref]
- Lee MW, Kang D, Lim HK, et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma <3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol 2020;30:2391-400. [Crossref] [PubMed]
- Santambrogio R, Barabino M, D'Alessandro V, et al. Micronvasive behaviour of single small hepatocellular carcinoma: which treatment? Updates Surg 2021;73:1359-69. [Crossref] [PubMed]
- Takahashi H, Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr 2020;9:49-58. [Crossref] [PubMed]
- Siperstein A, Garland A, Engle K, et al. Laparoscopic radiofrequency ablation of primary and metastatic liver tumors. Technical considerations. Surg Endosc 2000;14:400-5. [Crossref] [PubMed]
- Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005;242:158-71. [Crossref] [PubMed]
- Mimmo A, Pegoraro F, Rhaiem R, et al. Microwave Ablation for Colorectal Liver Metastases: A Systematic Review and Pooled Oncological Analyses. Cancers (Basel) 2022;14:1305. [Crossref] [PubMed]
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol 2015;25:3438-54. [Crossref] [PubMed]
- Birsen O, Aliyev S, Aksoy E, et al. A critical analysis of postoperative morbidity and mortality after laparoscopic radiofrequency ablation of liver tumors. Ann Surg Oncol 2014;21:1834-40. [Crossref] [PubMed]
- Foroutani A, Garland AM, Berber E, et al. Laparoscopic ultrasound vs triphasic computed tomography for detecting liver tumors. Arch Surg 2000;135:933-8. [Crossref] [PubMed]
- Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol 2010;17:171-8. [Crossref] [PubMed]
- Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol 2005;23:1358-64. [Crossref] [PubMed]
- Sindram D, Lau KN, Martinie JB, et al. Hepatic tumor ablation. Surg Clin North Am 2010;90:863-76. [Crossref] [PubMed]
- Santambrogio R, Barabino M, D'Alessandro V, et al. Laparoscopic thermoablation for hepatocellular carcinoma in patients with liver cirrhosis: an effective procedure for tricky tumors. Med Oncol 2020;37:32. [Crossref] [PubMed]
- Santambrogio R, Bianchi P, Pasta A, et al. Ultrasound-guided interventional procedures of the liver during laparoscopy: technical considerations. Surg Endosc 2002;16:349-54. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2014;21:723-31. [Crossref] [PubMed]
- Okumura S, Goumard C, Gayet B, et al. Laparoscopic versus open two-stage hepatectomy for bilobar colorectal liver metastases: A bi-institutional, propensity score-matched study. Surgery 2019;166:959-66. [Crossref] [PubMed]
- Fuks D, Nomi T, Ogiso S, et al. Laparoscopic two-stage hepatectomy for bilobar colorectal liver metastases. Br J Surg 2015;102:1684-90. [Crossref] [PubMed]
- Jiao LR, Hakim DN, Gall TM, et al. A totally laparoscopic associating liver partition and portal vein ligation for staged hepatectomy assisted with radiofrequency (radiofrequency assisted liver partition with portal vein ligation) for staged liver resection. Hepatobiliary Surg Nutr 2016;5:382-7. [Crossref] [PubMed]
- Liu Z, Ahmed M, Weinstein Y, et al. Characterization of the RF ablation-induced 'oven effect': the importance of background tissue thermal conductivity on tissue heating. Int J Hyperthermia 2006;22:327-42. [Crossref] [PubMed]
- Ahmed M, Liu Z, Afzal KS, et al. Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology 2004;230:761-7. [Crossref] [PubMed]
- Berber E, Siperstein AE. Perioperative outcome after laparoscopic radiofrequency ablation of liver tumors: an analysis of 521 cases. Surg Endosc 2007;21:613-8. [Crossref] [PubMed]
- Lencioni R, de Baere T, Martin RC, et al. Image-Guided Ablation of Malignant Liver Tumors: Recommendations for Clinical Validation of Novel Thermal and Non-Thermal Technologies - A Western Perspective. Liver Cancer 2015;4:208-14. [Crossref] [PubMed]
- Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol 2013;36:505-11. [Crossref] [PubMed]
- Fan W, Li X, Zhang L, et al. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol 2012;198:W46-50. [Crossref] [PubMed]
- Heerink WJ, Solouki AM, Vliegenthart R, et al. The relationship between applied energy and ablation zone volume in patients with hepatocellular carcinoma and colorectal liver metastasis. Eur Radiol 2018;28:3228-36. [Crossref] [PubMed]
- Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol 2014;21:4278-83. [Crossref] [PubMed]
- Nielsen K, van Tilborg AA, Meijerink MR, et al. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg 2013;37:1340-7. [Crossref] [PubMed]
- Agcaoglu O, Aliyev S, Karabulut K, et al. Complementary use of resection and radiofrequency ablation for the treatment of colorectal liver metastases: an analysis of 395 patients. World J Surg 2013;37:1333-9. [Crossref] [PubMed]
- Wakai T, Shirai Y, Sakata J, et al. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol 2008;15:2472-81. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25:S69-83. [Crossref] [PubMed]
- Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 2010;28:493-508. [Crossref] [PubMed]
- Benhaim L, El Hajjam M, Malafosse R, et al. Radiofrequency ablation for colorectal cancer liver metastases initially greater than 25 mm but downsized by neo-adjuvant chemotherapy is associated with increased rate of local tumor progression. HPB (Oxford) 2018;20:76-82. [Crossref] [PubMed]
- Aksoy E, Aliyev S, Taskin HE, et al. Clinical scenarios associated with local recurrence after laparoscopic radiofrequency thermal ablation of colorectal liver metastases. Surgery 2013;154:748-52; discussion 752-4. [Crossref] [PubMed]
- Mulier S, Ruers T, Jamart J, et al. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg 2008;25:445-60. [Crossref] [PubMed]
- Yang G, Wang G, Sun J, et al. The prognosis of radiofrequency ablation versus hepatic resection for patients with colorectal liver metastases: A systematic review and meta-analysis based on 22 studies. Int J Surg 2021;87:105896. [Crossref] [PubMed]
- Liu M, Wang K, Wang Y, et al. Short- and long-term outcomes of hepatectomy combined with intraoperative radiofrequency ablation for patients with multiple primarily unresectable colorectal liver metastases: a propensity matching analysis. HPB (Oxford) 2021;23:1586-94. [Crossref] [PubMed]
- Huang Z, Pan Y, Zhou P, et al. Long-term outcomes of ultrasound-guided percutaneous microwave ablation versus resection for colorectal cancer liver metastases: a propensity-score matched study. Int J Hyperthermia 2021;38:1276-84. [Crossref] [PubMed]
- Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009;197:728-36. [Crossref] [PubMed]
- Kim KH, Yoon YS, Yu CS, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc 2011;81:25-34. [Crossref] [PubMed]
- Oshowo A, Gillams A, Harrison E, et al. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 2003;90:1240-3. [Crossref] [PubMed]
- Hof J, Wertenbroek MW, Peeters PM, et al. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg 2016;103:1055-62. [Crossref] [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 466-7. [Crossref] [PubMed]
- McKay A, Fradette K, Lipschitz J. Long-term outcomes following hepatic resection and radiofrequency ablation of colorectal liver metastases. HPB Surg 2009;2009:346863. [Crossref] [PubMed]
- Jasarovic D, Stojanovic D, Mitrovic N, et al. Resection or radiofrequency ablation of colorectal liver metastasis. Vojnosanit Pregl 2014;71:542-6. [Crossref] [PubMed]
- Faitot F, Faron M, Adam R, et al. Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases: a case-matched analysis of surgical and oncological outcomes. Ann Surg 2014;260:822-7; discussion 827-8. [Crossref] [PubMed]
- Meijerink MR, Puijk RS, van Tilborg AAJM, et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol 2018;41:1189-204. [Crossref] [PubMed]
Cite this article as: Barabino M, Piccolo G, Opocher E. Laparoscopic thermal ablation for colorectal liver metastases: technical tips and pitfalls. Laparosc Surg 2022;6:19.


