
Managing complex pancreatic anastomoses after minimally invasive pancreaticoduodenectomy
Introduction
Several pancreatic anastomoses (PAs) types have been described after mini-invasive pancreaticoduodenectomy (MIPD). These seek to reduce the incidence of post operative pancreatic fistula (POPF) (1-5). In most cases, the choice of the PA is mainly driven by the surgeon’s experience and habits. However, in some situations, the selection of the PA should be driven either by the type of pancreatic stump or by the high comorbidity of the patient. The main features of the pancreatic stump contributing to complex PAs include the presence of a bulky-fatty pancreatic stump, the absence of a visible main pancreatic duct on the pancreatic surface, presence of ongoing acute pancreatitis of the pancreatic stump, or in POPF cases requiring reintervention where an anastomosis could be considered. All these situations require a case-by-case analysis of the surgical strategy.
In the following sections, we describe strategies to be adopted to deal with such complex PAs after MIPD, focusing on both pancreatic stump complexity and/or associated comorbidities.
Managing PAs with complex pancreatic remnant
Fatty-bulky pancreas
Obesity and an increasingly aged population are the main factors associated with the presence of the fatty pancreas (6-8), which increases the risk of POPF. The amount of fatty tissue in the pancreatic remnant can be evaluated preoperatively by magnetic resonance imaging (MRI) or computed tomography (CT) scan (9,10) (Figure 1A).
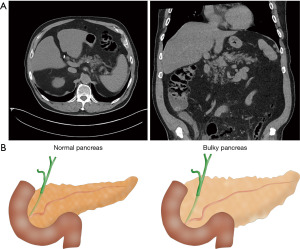
A fatty pancreas is frequently associated with a bulky pancreatic stump. In such cases, the diameter of the pancreatic stump exceeds the height of the small bowel, which precludes performing some of the most common pancreaticojejunostomies (PJ), such as Blumgart, Peng, etc. (Figure 1B). Pancreaticogastrostomy (PG) is an alternative to PJ for fatty-bulky pancreas.
In Video 1 we show a case of fatty-bulky pancreas. In the video we also describe a few technical details pivotal to achieving a safe PG: (I) the mobilization of the pancreatic stump from both the splenic vein and artery should be enough to avoid the so called “Pisa Tower Effect”: when the angle between the pancreatic surface and splenic vessels is smaller than 90 degrees, case in which the PG anastomosis is subject to excessively imbalanced tensions; (II) the incision on the posterior wall of the stomach should be of the same length of the stump to facilitate the intussusception; (III) a purse-string technique should be adopted avoiding stitches which can easily tear the pancreatic stump, as shown in the video.
No visible main pancreatic duct
Anatomical variations and developmental anomalies of the pancreas and pancreatic duct can lead to differences in the duct’s course despite most of these variations do not cause symptoms (11-13) (Figure 2).
Pancreaticoduodenectomies (PDs) are sometimes performed for benign or border-line tumors such as endocrine or ampullary tumors. In these cases, the main pancreatic duct may be thin and sometimes not visible on the remaining pancreatic stump. In rare instances, the duct could be absent or duplicated (11-13). In all these situations, a duct-to-mucosa PJ (classical or modified Blumgart) cannot be performed, requiring the surgeon to consider alternatives like dunking PJ (1) or PG. Both of these techniques involve the invagination of a portion of the remaining pancreas into the small intestine or stomach, eliminating the need for a formal anastomosis on the Wirsung duct.
Acute pancreatitis of pancreatic stump
Acute pancreatitis has been reported as an intraoperative finding during PD. In some cases, after completing the resection phase of PD, an inspection of the remaining pancreas and nearby tissues reveals areas of steatonecrosis and/or noticeable swelling and inflammation, especially in those cases where several preoperative attempts of endoscopic retrograde cholangiopancreatography have been performed.
There is no definitive evidence-based guidance on whether to perform a PA or a total or near total pancreatectomy in such critical and uncommon challenging situations. It is important, however, to weigh the consequences of such an aggressive treatment, including severe comorbidities such as brittle diabetes. The decision should be based on the surgeon’s experience and evaluated on a case-by-case basis. The second video herein presented (Video 2) shows a patient with a bulky and fatty pancreas stump with signs of acute pancreatitis on the stump. In this case, a total pancreatectomy was finally performed.
Nowadays it is still under debate whether total pancreatectomy is superior to PA. However, in our experience and opinion, in such challenging cases total pancreatectomy represents an effective alternative, decreasing the early post operative morbidity-mortality.
Failure of previously performed PA
A failure of a previous PA can happen in two different situations: (I) during re-operation, due to severe POPF or postoperative bleeding associated with it; or (II) during surgery when the PA looks clearly not safe and effective.
First, in the case of reoperation, rarely a re-anastomosis is feasible (12-23). In most cases, a total or sub-total pancreatectomy is the preferred strategy. In the case of intra-operative failure of PA, the options are: (I) re-cutting the edge of the pancreatic stump and, after further mobilization from the splenic vessels, re-do the PA; (II) near total pancreatectomy or total pancreatectomy.
Recently, Ielpo et al. (17) have shown encouraging results with radiofrequency ablation of the main pancreatic duct in case of failure of PA, in both scenarios, during surgery o during reoperation. The latter approach has the potential to become a good alternative to pancreatic totalization in the complex cases discussed here as they seem to preserve the islets of Langerhans, and consequently the endocrine function.
Managing high-morbidity patients
Grade C POPF remains the primary cause of severe postoperative complications after PD, with mortality related mainly to the “failure to rescue” concept. This idea emphasizes that timely and effective treatment of postoperative complications can prevent death. Moreover, the development of Grade C POPF in patients with high comorbidity (e.g., obesity, cardiopathy, sarcopenia) increases the risk of postoperative death. Various scoring systems can predict POPF occurrence aiming to identify high-risk cases (15,16). If a high risk for POPF is found in a patient with high comorbidity, the surgeon should consider performing a total pancreatectomy (18,19).
Once again, there are two possible scenarios: (I) the decision to perform a total pancreatectomy is made preoperatively; or (II) the decision is made intraoperatively after evaluating the quality of the pancreatic stump, bleeding, and the challenges encountered during the procedure. In the first scenario, a total spleen-preserving pancreatectomy may be recommended, while in the second scenario, a classical total pancreatectomy seems more appropriate as shown in the last video (Video 3).
Finally, early experimental results using preoperative endoluminal radiofrequency ablation in animal models indicate a reduction in POPF preserving the endocrine function (24). Thus again, endoluminal radiofrequency ablation bears great promise as a complementary tool in these complex cases.
Conclusions
Pancreatic surgeons often encounter complicated situations during PAs that require unconventional approaches. This article presents various alternative strategies (summarized in Table 1) that can help handle the most challenging cases providing multimedia support illustrating such strategies in detail.
Table 1
| Intraoperative findings | Suggested approach |
|---|---|
| Fatty-bulky pancreas | Pancreaticogastrostomy |
| No visible main pancreatic duct | Dunking pancreaticojejunostomy |
| Pancreaticogastrostomy | |
| Acute pancreatitis of pancreatic remnant | Total pancreatectomy |
| Failure of previously performed pancreatic anastomosis | Re-do the pancreatic anastomosis |
| Near total pancreatectomy | |
| Total pancreatectomy | |
| Radiofrequency ablation of the main pancreatic duct | |
| High-morbidity patients/high-risk for POPF | Total pancreatectomy with/without splenic preservation |
| Radiofrequency ablation of the main pancreatic duct |
POPF, post operative pancreatic fistula.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://ls.amegroups.com/article/view/10.21037/ls-23-15/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ls.amegroups.com/article/view/10.21037/ls-23-15/coif). B.I. serves as an unpaid editorial board member of Laparoscopic Surgery from November 2022 to October 2024. E.R. serves as an unpaid editorial board member of Laparoscopic Surgery from October 2023 to September 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this article were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim EY, You YK, Kim DG, et al. A simple pancreaticojejunostomy technique for hard pancreases using only two transpancreatic sutures with buttresses: a comparison with the previous pancreaticogastrostomy and dunking methods. Ann Surg Treat Res 2016;90:64-71. [Crossref] [PubMed]
- Kawaida H, Kono H, Hosomura N, et al. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol 2019;25:3722-37. [Crossref] [PubMed]
- Ricci C, Casadei R, Taffurelli G, et al. Minimally Invasive Pancreaticoduodenectomy: What is the Best "Choice"? A Systematic Review and Network Meta-analysis of Non-randomized Comparative Studies. World J Surg 2018;42:788-805. [Crossref] [PubMed]
- Fiorentini G, Tamburrino D, Belfiori G, et al. Which is the best pancreatic anastomosis? Minerva Chir 2019;74:241-52. [Crossref] [PubMed]
- Wang W, Zhang Z, Gu C, et al. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: A network meta-analysis of randomized control trials. Int J Surg 2018;57:111-6. [Crossref] [PubMed]
- Rosso E, Casnedi S, Pessaux P, et al. The role of "fatty pancreas" and of BMI in the occurrence of pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg 2009;13:1845-51. [Crossref] [PubMed]
- Sugimoto M, Takahashi S, Kojima M, et al. In Patients with a Soft Pancreas, a Thick Parenchyma, a Small Duct, and Fatty Infiltration Are Significant Risks for Pancreatic Fistula After Pancreaticoduodenectomy. J Gastrointest Surg 2017;21:846-54. [Crossref] [PubMed]
- Gaujoux S, Cortes A, Couvelard A, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 2010;148:15-23. [Crossref] [PubMed]
- Hanaki T, Uejima C, Amisaki M, et al. The attenuation value of preoperative computed tomography as a novel predictor for pancreatic fistula after pancreaticoduodenectomy. Surg Today 2018;48:598-608. [Crossref] [PubMed]
- Tranchart H, Gaujoux S, Rebours V, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg 2012;256:139-45. [Crossref] [PubMed]
- Adibelli ZH, Adatepe M, Imamoglu C, et al. Anatomic variations of the pancreatic duct and their relevance with the Cambridge classification system: MRCP findings of 1158 consecutive patients. Radiol Oncol 2016;50:370-7. [Crossref] [PubMed]
- Türkvatan A, Erden A, Türkoğlu MA, et al. Congenital variants and anomalies of the pancreas and pancreatic duct: imaging by magnetic resonance cholangiopancreaticography and multidetector computed tomography. Korean J Radiol 2013;14:905-13. [Crossref] [PubMed]
- Kawasaki S, Itoi T, Iwasaki E, et al. Successful Pancreatic Duct Stent Placement for Recurrent Pancreatitis in a Patient with Polysplenia with Agenesis of the Dorsal Pancreas and Peutz-Jeghers Syndrome. Intern Med 2016;55:1743-6. [Crossref] [PubMed]
- Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015;386:85-96. [Crossref] [PubMed]
- Lee B, Yoon YS, Kang CM, et al. Fistula risk score-adjusted comparison of postoperative pancreatic fistula following laparoscopic vs open pancreatoduodenectomy. J Hepatobiliary Pancreat Sci 2021;28:1089-97. [Crossref] [PubMed]
- Perri G, Marchegiani G, Partelli S, et al. Preoperative risk stratification of postoperative pancreatic fistula: A risk-tree predictive model for pancreatoduodenectomy. Surgery 2021;170:1596-601. [Crossref] [PubMed]
- Ielpo B, Pueyo-Périz EM, Radosevic A, et al. Clinical case report: endoluminal thermal ablation of main pancreatic duct for patients at high risk of postoperative pancreatic fistula after pancreaticoduodenectomy. Int J Hyperthermia 2021;38:755-9. [Crossref] [PubMed]
- Salvia R, Lionetto G, Perri G, et al. Total pancreatectomy and pancreatic fistula: friend or foe? Updates Surg 2021;73:1231-6. [Crossref] [PubMed]
- Luu AM, Olchanetski B, Herzog T, et al. Is primary total pancreatectomy in patients with high-risk pancreatic remnant justified and preferable to pancreaticoduodenectomy? -a matched-pairs analysis of 200 patients. Gland Surg 2021;10:618-28. [Crossref] [PubMed]
- Muaddi H, Karanicolas PJ. Postoperative pancreatic fistula: Still the Achilles' heel of pancreatic surgery. Surgery 2021;169:1454-5. [Crossref] [PubMed]
- Marchegiani G, Bassi C. Prevention, prediction, and mitigation of postoperative pancreatic fistula. Br J Surg 2021;108:602-4. [Crossref] [PubMed]
- Smits FJ, Molenaar IQ, Besselink MG, et al. Early recognition of clinically relevant postoperative pancreatic fistula: a systematic review. HPB (Oxford) 2020;22:1-11. [Crossref] [PubMed]
- Kushiya H, Nakamura T, Asano T, et al. Predicting the Outcomes of Postoperative Pancreatic Fistula After Pancreatoduodenectomy Using Prophylactic Drain Contrast Imaging. J Gastrointest Surg 2021;25:1445-50. [Crossref] [PubMed]
- Andaluz A, Ewertowska E, Moll X, et al. Endoluminal radiofrequency ablation of the main pancreatic duct is a secure and effective method to produce pancreatic atrophy and to achieve stump closure. Sci Rep 2019;9:5928. [Crossref] [PubMed]
Cite this article as: Vellalta Muxí G, Ielpo B, Anselmo A, de Blasi V, Rosso E. Managing complex pancreatic anastomoses after minimally invasive pancreaticoduodenectomy. Laparosc Surg 2024;8:1.



